(1)
Department of Medicine, University of Miami Hospital, Miami, FL, USA
(2)
Cardiac Unit, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Abstract
A patent foramen ovale (PFO) is highly prevalent among the adult population and has been associated to cryptogenic strokes. The diagnosis of PFO is done through echocardiographic studies. Due to the high prevalence of PFO, the identification of “high risk” features such as atrial septal aneurysm (ASA) becomes extremely important in the management of these patients. Currently, the major controversy regarding the treatment and secondary prevention of patients with cryptogenic stroke and PFO is based on the fact that most of the studies investigating medical and interventional based therapies, failed to include patients with “high risk” features.
Keywords
Patent foramen ovaleAtrial septal aneurysmTranscatheter PFO closure devicesEustachian valvesIntroduction
A patent foramen ovale (PFO) is highly prevalent among the adult population. It allows shunting of blood through the inter-atrial septum and has been associated with cryptogenic stroke and migraines with aura. Currently, echocardiography is the most important diagnostic tool, and, the sensitivity and specificity of the study depends on the modalities available: transthoracic (TTE), transesophageal (TEE) and transcranial Doppler (TCD), as well as the use of agitated saline and the site of injection.
It has been over 140 years since the controversy regarding the potential cause-effect relationship between a PFO and cryptogenic strokes was originally proposed by Dr. Julius Cohnheim [1]. Since then, multiple attempts have tried to prove that closure of PFO could be an effective therapy to prevent subsequent neurological events. Medical therapy alone seems to be appropriate for patients with “low risk anatomy” but those with “high risk” features might not obtain sufficient protection. Transcatheter (TC) PFO closure has been shown in observational and prospective studies to be a safe and efficient therapy. However, the results of multiple randomized clinical trials (RCT) have failed to show significant benefits from catheter closure, mostly due to the fact that each study was underpowered. Thus, meta-analysis of the combined studies is important. The fact that there are no randomized clinical trials studying the impact of TC-PFO closure versus standardized medical therapy in patients with “high risk” anatomy PFO, contributes to the controversy surrounding secondary prevention in patients with cryptogenic stroke and PFO.
The purpose of this chapter is to provide a review of different aspects regarding PFO and neurological syndromes, including anatomy, diagnosis and therapeutic options, as well as the data supporting these different strategies.
Embryology and Anatomy
The development of the foramen ovale is critical in the embryological development of the heart. Approximately at the 5th week of development, a very thin septum primum begins to migrate downwards towards the endocardial cushion. The hiatus in between both structures forms the foramen primum. As the migration of the septum primum continues, apoptotic changes within the septum will originate the foramen secundum. On the right atrial surface of the foramen secundum a more muscular and thicker septum secundum migrates downwards covering the foramen secundum and leaving a small foramen on the bottom of the atrium, the foramen ovale. This structure will provide a right to left shunt necessary for fetal circulation. After birth, this communication will spontaneously close in approximately 75 % of the population [2].
The anatomy of the PFO is highly variable and may be associated with a long tunnel of >10 mm, an atrial septal aneurysm which is a redundancy of the atrial septum of over 15 mm, a hypermobile septum or a persistent Eustachian valve.
Prevalence
Factors Associated with Paradoxical Embolization
The PFO in Cryptogenic Stroke Study (PICSS) was a multicenter study that evaluated TEE findings in patients randomly assigned to warfarin or aspirin in the Warfarin-Aspirin Recurrent Stroke Study (WARSS). PICSS found that patients with cryptogenic stroke had a significantly higher incidence of a large PFO when compared to those patients having a stroke of known cause (20 % vs. 9.7 % p < 0.001) [6]. Moreover, Steiner et al. performed TEE in 95 patients with a first ischemic stroke over 39 years of age [7]. The stroke subtype and MRI/CT imaging data were evaluated blinded to the presence of a PFO. These findings were compared between two groups: patients with a medium to large PFO (>2 mm) and small (<2 mm) or no PFO. Stroke patients with larger PFOs showed more brain imaging features of embolic infarcts than those with small PFOs.
The presence of a prominent Eustachian valve (EV) has been proposed as responsible for re-directing blood flow towards the septum, potentially allowing emboli to travel through the inter-atrial septum into the left atrium. This hypothesis was evaluated with TEE by Schuchlenz et al. by comparing patients who had cryptogenic strokes to healthy volunteers and found a significantly higher incidence of PFO and EV in those patients with cryptogenic stroke [8].
The PFO and ASA study group followed 581 ischemic stroke patients under the age of 55 years of age. The patients were started on aspirin within 3 months of their neurological event, and followed up for a period of 4 years. The patients were divided into groups depending of the characteristics of the inter-atrial septum. Mas et al. found that the presence of both atrial septal abnormalities (PFO and ASA) was a significant predictor of increased risk of recurrent cerebrovascular events, whereas the presence of a PFO alone or an ASA alone was not [9]. Moreover, their finding suggested that aspirin as secondary prevention for recurrent events may not be enough for this subgroup of patients. These findings are in agreement with findings of other studies, especially in patients with right-to-left shunting at rest [10]. Stone et al. followed prospectively a group of stroke patients found to have a PFO during TEE and divided them into “large” degree shunting (≥20 microbubbles) and “small” degree shunting (≥3 but <20 microbubbles). Patients with “large” shunts had a 31 % incidence of a recurrent event versus none in the “small” shunt group despite the use of antiplatelet and/or anticoagulation. Therefore, patients with “large” shunts, should be considered at a significantly higher risk for subsequent adverse neurologic events [11]. It has also been proposed that “long-tunnel” PFO anatomy represents an environment fertile for clot formation, with subsequent embolization. However, there is no clear evidence supporting this hypothesis [12].
In a venography study, Stollberger et al. presented evidence that patients with ischemic stroke due to suspected paradoxical embolization have a higher incidence of deep venous thrombosis [13]. Therefore, conditions that facilitate the formation of deep venous thrombosis deserve special attention when evaluating patients with PFO and cryptogenic stroke. The May-Thurner syndrome, in which the right common iliac artery compresses the overlying left common iliac vein has been found to have a higher incidence in patients with PFO-related stroke [14, 15]. In a prospective study of patients with large pulmonary embolism, it was found that those patients with PFO had a sixfold higher risk of stroke when compared to those without PFO [16] Table. 6.1.
Table. 6.1
“High risk” features of a PFO increasing stroke risk
PFO associated with an ASA |
Large size of the PFO |
PFO associated with a prominent EV |
PFO associated with a large degree of shunt |
PFO Diagnosis
It is very important to remember when evaluating a patient for the presence of a PFO, that a PFO is present in approximately 25 % of the general healthy population [3]. Thus, it is important to be mindfull of the clinical presentation of every particular case. Moreover, the clinician should be able to identify particular “high risk” features that might make the presence of a PFO more relevant (Table. 6.1).
There are different modalities available for the diagnosis of PFO. The most commonly used are TTE, TEE and TCD coupled with agitated saline injection in association with the Valsalva manuever. The most common initial modality is TTE for evaluation of cardiac sources of emboli. Agitated saline contrast increases the diagnostic sensitivity by enhancing echocardiographic detection of the trivial intermittent right-to-left shunting across the PFO. However, the sensitivity of TEE is higher than TTE despite the use of agitated saline [3]. Hamman et al. [17] demonstrated increased sensitivity when the injection of agitated saline was performed from the femoral vein versus the traditional antecubital vein. This is probably due to the fact that the bubbles ascending through the inferior vena cava will encounter the EV and flow preferentially towards the septum. These findings were more evident when using TEE and TCD versus TTE.
Medical Therapy for Secondary Prevention of Cryptogenics Strokes
Medical therapy for secondary prevention in cryptogenic strokes continues to be the most common initial approach for patients after the initial neurological event. However, the type of medical therapy has been loosely defined in different studies and there is no consensus regarding the use of either antiplatelet agents or anticoagulation. Furthermore, there is no agreement regarding an escalation in therapy for patients with “high risk” PFO anatomical features.
WARSS, was the first randomized controlled study to compare the effect of warfarin and aspirin after prior non-cardioembolic ischemic stroke. WARSS showed that aspirin was as effective as warfarin in prevention of stroke recurrence, but the presence of a PFO was not specifically systematically evaluated [18]. Moreover, WARSS showed that the incidence of death and recurrent ischemic strokes was similar in both groups of patients (Warfarin vs. Aspirin). However, the incidence of recurrent ischemic strokes was equally high (17.8 % vs. 16.0 % for the Warfarin and the Aspirin groups respectively, p = 0.2). Of interest that same year in the same journal, the report from the PFO and ASA study group [9] was published. In their prospective study of cryptogenic stroke patients treated with aspirin, when the PFO was associated with an ASA, aspirin was not as effective for secondary prevention. A year later, a substudy of WARSS, the PICCS trial compared secondary prevention with aspirin versus warfarin. In the cohort of patients with cryptogenic stroke, there was a trend towards fewer neurological events in the arm treated with warfarin when a PFO was present [6]. The evidence seems to indicate that warfarin might be more appropriate for secondary prevention in patients with “high risk” PFO. However, this was associated with an increase in bleeding complications. New anticoagulation agents are now available, and it will be interesting to see how the use of this new medication class will impact the secondary prevention of cryptogenic strokes in patients with a PFO, especially those with “high risk” features. Studies are on going.
Surgical Therapy for Secondary Prevention of Cryptogenic Strokes
Surgical closure of PFO has shown good results, with a low incidence of recurrent events [3]. However, due to the invasive nature of the intervention, it is not a commonly used therapy. Currently, it is reserved for cases that will require surgical intervention for another condition or in those in whom percutaneous closure can not be performed related to the inter-atrial septal anatomy.
PFO-TC Closure for Secondary Prevention of Cryptogenic Strokes
Although there are no FDA approved devices for TC-PFO closure in the United States, there is a vast experience using off-label devices for secondary prevention. Reports have shown that TC-PFO closure is a safe intervention that is associated with favorable short- and intermediate-term outcomes [19]. Moreover, studies show excellent long-term outcomes when used for secondary prevention in patients with cryptogenic stroke (Fig. 6.1) [20]. A systematic review and meta-analysis of observational studies showed the annual rate of strokes after PFO-closure is approximately 0.3–0.8 %, lower than the 1.98–5.0 % in the medical group [3, 21]. This translates into an 84 % reduction in the rate of recurrent neurological events when compared to medical management alone.
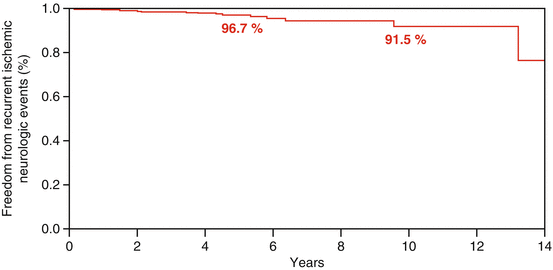

Fig. 6.1
Kaplan-Mayer recurrent neurological event after TC-PFO closure
A prospective study with long term follow-up showed that the presence of a substantial residual shunt after TC-PFO closure was an important predictor of recurrent neurological events with a relative risk of 4.2 [22]. Therefore, the use of a second device for secondary prevention of recurrent neurological events has been an important clinical question. In a retrospective study by Diaz et al., 424 patients with at least a 5 % substantial residual shunt found that the placement of a second device was safe and effective in treating the residual shunt. Moreover, there were no neurological events at a mean follow-up of 3 years. However, the clinical significance of treating residual shunts with a second device would be at least difficult to prove, since the event rate is low even with untreated PFOs [23].
However, results from three recently published RCTs failed to show a significant benefit of TC PFO closure over medical therapy [24–26]. One trial utilized the StarFlex device [24], the other two studied the Amplatzer device [25, 26]. The main limitation of all three RCTs was the small number of events during follow-up, raising the possibility of a “type 2 error” (failure to detect a true difference between treatments due to lack of power). This lack of power can be explained by the difficulties in enrolling patients in a trial, while the study device was available as an off-label therapy. Another important observation about the three RCTs was the inclusión of relatively “low risk” PFOs into their analysis [24–26]. The presence of an ASA in patients included in the RCTs, a feature that has been associated with a higher incidence of recurrent neurological events, ranged from only 23–26 %.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


