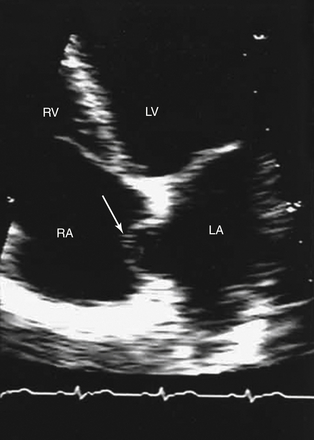
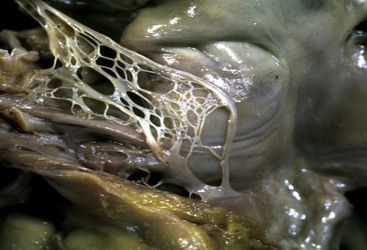
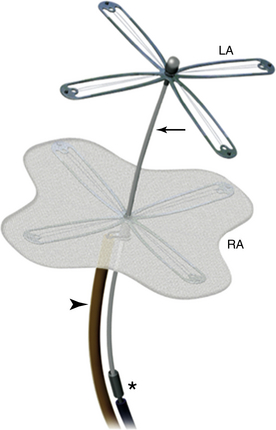
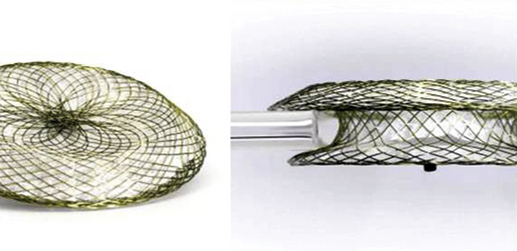
Chapter 20 The foramen ovale is pivotal during intrauterine life and facilitates the passage of oxygenated blood from the placenta to the fetal circulatory system. The blood flow from the umbilical veins enters the right atrium through the inferior vena cava (IVC) and bypasses the nonfunctional lungs across the foramen to enter the systemic circulation. Most blood flow from the superior vena cava (SVC) is routed through the tricuspid valve and enters the right ventricle. At birth, right heart pressure and pulmonary vascular resistance (PVR) drop, and there is increased pulmonary blood flow. Left atrial pressure may also rise as pulmonary venous return increases and there is a decrease in left ventricular compliance. Either or both of these mechanisms may result in functional closure of the foramen ovale by apposition of the septum primum against the septum secundum (Figure 20–1). In most people, the caudal portion of the septum primum on the left side and the cranial portion of the septum secundum on the right side fuse permanently in the ensuing months.1 In 25% of the population, both parts of the interatrial septum remain separable.2 The patent foramen ovale (PFO) is positioned caudally in the septum secundum and cranially in the septum primum, forming a slit valve, and the channel may open either when the right atrial pressure overrides the left atrial pressure (e.g., during a Valsalva maneuver), or merely from the inferior venacaval blood stream directed towards the fossa ovalis. This could provide an opportunity for small, otherwise undetectable clots to cross into the arterial system, bypassing the filter of the pulmonary vasculature and leading to a paradoxical embolism. Figure 20–1 Patent forman ovale (PFO). The incidence of a PFO decreases with age, whereas the average diameter slightly increases. This could be because there may be closure of small PFOs later in life. The prevalence of PFO declines from 34% during the first three decades, to 25% during the fourth to eighth decade, and to 20% in the ninth decade and beyond.2 Selective mortality or spontaneous closure even late in life may be reasons for the decrease. An autosomal dominant inheritance has been demonstrated. There is no gender preference.3 Atrial septal aneurysm (ASA) is a congenital abnormality of the interatrial septum characterized by a redundant, amuscular membrane in the region of the fossa ovalis that corresponds to a sector of the central part of the septum primum (Figure 20–2).4,5 The prevalence of ASA in the general population was about 1% in autopsy series and 2.2% in a population-based transesophageal echocardiography (TEE) study.6,7 ASA is associated with a PFO in 50% to 85% of cases. ASA is generally diagnosed if the diameter of the base of the flimsy portion of the interatrial septum exceeds 15 mm and if the excursion of the aneurysmal membrane exceeds 10 mm in either the left or right atrium, or if the sum of the total excursion is greater than 10 mm.8 ASA has been hypothesized to act as facilitator for paradoxical embolism via the following mechanisms: (1) by increasing the PFO diameter because of the highly mobile atrial septal tissue, leading to a more frequent and wider opening of an otherwise small channel9; (2) promoting right-to-left shunting by redirecting flow from the IVC toward the PFO10; and (3) acting as a nidus for local thrombus formation with subsequent embolization.11 A Chiari network is an embryologic remnant of the right venous sinus valve (Figure 20–3).12,13 In a study of 1436 consecutive adult patients, there was a Chiari network prevalence of 2% with a common association between Chiari networks and PFO in 83% of patients. Large right-to-left shunting was found significantly more often in patients with Chiari networks than in patients who were part of a control group (55% vs. 12%, p = 0.001). This study also found Chiari networks associated with ASA in 24% of patients. The Chiari network is more common in cryptogenic stroke patients than in patients evaluated for other indications (4.6% vs. 0.5%), and it may also facilitate paradoxical embolism.14 A number of ultrasound-guided options are available to diagnose a PFO. Imaging modalities such as contrast-enhanced transmitral Doppler, transthoracic echocardiography (TTE), TEE, and transcranial Doppler are established methods to detect a PFO with an active right-to-left shunt.15 TTE and TEE are also able to detect potential cardiac sources of embolism other than PFO, including benign cardiac tumors (myxoma, papillary fibroelastoma), valvular strands (Lambl excrescences), mitral valve prolapse, left atrial spontaneous echo contrast or left atrial appendage thrombus, vegetations, and aortic arch and thoracic aortic atheroma. Imaging should be repeated until the operator is confident that the existence of a PFO has been ruled out. Given the importance of coordinating these factors for diagnosing a PFO, bubble contrast studies, in which no sedation is necessary, are initially performed during TTE. Bubble contrast echocardiography requires an experienced operator who is aware of the potential pitfalls of misdiagnosis, which include the difficulty in distinguishing a pulmonary-level shunt from a cardiac-level shunt. Contrast from a cardiac shunt usually appears within three cardiac cycles. Various classification schemes have been proposed to standardize the grading of the size of the shunt according to the number of bubbles shunting, although none are, as yet, predominant. If analysis with TTE bubble contrast shows a right-to-left shunt, TEE is necessary to confirm the presence and define the anatomy of the PFO, as well as to exclude the presence of other potential shunts.16 During the diagnostic work-up in patients with cryptogenic stroke and presumed paradoxical embolism, the diagnosis of PFO is or has usually been made with contrast TEE echocardiography. The sensitivity of TTE for detection of a PFO is lower. Therefore the assessment of the number of high-intensity transient signals by means of transcranial Doppler recordings is highly sensitive for right-to-left shunting but is not as specific as transesophageal contrast echocardiography.17–19 Contrast transcranial Doppler ultrasonography is a highly sensitive and noninvasive method to screen for right-to-left shunting and shows close agreement with contrast echocardiography in shunt detection. This technique uses ultrasound to quantify the number of bubbles that reach the cerebral circulation. Patients with microemboli detected with contrast transcranial Doppler ultrasonography are more likely to have a history of cerebral ischemia. However, transcranial Doppler ultrasonography does not provide the anatomic information that can be derived from echocardiography and therefore cannot be used in isolation.20 Alternative methods for the detection of PFO include computed tomography (CT) and magnetic resonance imaging (MRI). These techniques are likely to be as specific as TEE but far more expensive and not as readily available.21,22 Several observations have confirmed the concept of a thrombus trapped in the PFO tunnel and its ability to embolize to the brain, as in the first report by Cohnheim in 1877.23 Zahn first used the term paradoxical embolus in 1881 to describe a branched thrombus from a uterine vein caught in a PFO on a postmortem examination.24 Paradoxical embolism is proposed as a mechanism for cryptogenic stroke and occurs when a thrombus from the systemic venous circulation passes to the systemic arterial circulation via a right-to-left shunt, usually a PFO. A stroke is considered “cryptogenic” when no source of embolism is detectable.25 This may be the case in up to 40% of strokes in young adults. In adults with cryptogenic strokes who are younger than 55 years of age, a PFO is found with a higher prevalence than in patients age 55 years and older. Retrospective analysis suggested an association between PFO, cryptogenic stroke, and young age. Some questions are unresolved: cryptogenic stroke is a complex diagnosis, not just related to PFO. A high percentage of the young and healthy population has a PFO without stroke. The association of both pathologies, PFO and cryptogenic stroke, may be incidental and is no proof that PFO is the cause of stroke.26–28 In a meta-analysis of case-control studies, the association between ischemic stroke and PFO was confirmed. In the same overview, the role of an ASA was assessed. An ASA is 10 times less common than a PFO. Initially, an ASA was considered a risk factor for left-sided embolic events.29 Recently, however, the Stroke Prevention Assessment of Risk in a Community study and the data of the Patent Foramen Ovale and Atrial Septal Aneurysm Study Group proved that an ASA enhances the risk of recurrent cerebrovascular events in the presence of a PFO but does not represent an independent source of embolism.30 The latter study also challenged the role of a PFO as a mediator of paradoxical emboli in general. Coagulation disorders, such as prothrombotic genetic polymorphisms (factor V Leiden mutation, protein C or S deficiencies, anticardiolipin antibodies, and prothrombin G20210A mutation) or thrombocytosis may also play a role in the pathogenesis of paradoxical embolism. The platypnea–orthodeoxia syndrome (POS) comprises both dyspnea (platypnea) and arterial desaturation (orthodeoxia) in the upright position with improvement in the supine position. It is uncommon, but several dozen cases are reported. Two components must coexist to create this syndrome. One is an anatomic defect, and the other is functional. The anatomic component must consist of an interatrial shunt, such as an atrial septal defect (ASD), PFO, fenestrated ASA, or intrapulmonary shunting. A functional component induces the deformity at the atrial level and may occur while the patient is rising to an upright from a recumbent position. Cardiac causes also exist, including pericardial effusion, constrictive pericarditis, and toxicity from drugs such as amiodarone. In this syndrome there is elevation of right atrial pressure, causing right-to-left shunt. Interestingly, blood may flow from right to left at the atrial level even when right heart pressure is normal, as typically occurs with persistent eustachian valves. Diagnosis of PFO with POS should be by tilt test, measuring arterial saturation in different positions, and contrast echocardiography, which should show intracardiac shunting. Note that the majority of right-to-left shunts in POS may be derived from IVC blood flow, and injection from an antecubital vein with return via the SVC may show less prominent shunting. The definitive treatment for POS is closure of the atrial shunt.31,32 Arterial gas embolism through an ASD was reported first in a scuba diver in 1986. Type 1 decompression sickness (DCS) is composed of localized joint pain, musculoskeletal pain, and skin rash, and type 2 DCS consists of neurologic symptoms (limb tingling, paresthesias, severe headache with mental confusion, paraplegia, loss of consciousness, audiovestibular symptoms, and dyspnea with chest pain). The PFO at rest is significantly associated with type 2 DCS. A recent study found a strong relationship between PFO size and DCS in a study of 230 divers. Another study demonstrated the functional and anatomic characteristics of PFO with and without DCS. This study suggested that DCS was associated with right-to-left shunting at rest. Atrial septal mobility and PFO diameter are also associated with the risk of developing DCS.33–35 There are no society guidelines governing the role of PFO closure in divers, and the decision to proceed should be based on best clinical judgment. Migraine and vascular headache may be related to PFO, according to an interesting series of studies. Migraines affect approximately 13% of the population aged 20 to 64 years, with 36% of migraines preceded by aura. Migraine with aura is associated with PFO and other causes of right-to-left shunting. It is hypothesized that a bloodborne substance that would ordinarily be filtered out by the lungs is delivered to the cerebral circulation via the shunt. However, the mechanisms that trigger migraines are unknown, and this theory remains unproven. Patients undergoing PFO closure for nonmigraine indications (e.g., paradoxical emboli) have reported an improvement in their migraine symptoms.36 Migraine is a risk factor for cryptogenic stroke, especially in young patients without atherosclerotic risk factors.36–38 The main indication for PFO closure is the prevention of recurrent paradoxical embolic cryptogenic stroke in younger patients (<55 years). This is done after an extensive work-up, including a complete neurologic examination and screening blood tests for thrombophilia. The American Academy of Neurology considers that evidence is insufficient for them to take a position on the efficacy of percutaneous or surgical closure, and American Heart Association (AHA)/American Stroke Association guidelines consider data insufficient to recommend PFO closure in patients with a first episode but recommend considering closure in patients who, although receiving medical treatment, experience a second episode (class IIb, evidence C).39,40 In the absence of abnormal results of several tests, including cerebral MRI (except for ischemic lesions), angio-MRI of the circle of Willis, echo Doppler of extracranial cervical arteries, 12-lead electrocardiograph, 24-hour Holter monitoring, and echocardiography, controversial data are available in the literature about PFO closure for cryptogenic stroke prevention.41 However, patient selection criteria, duration of follow-up study, definition of cryptogenic stroke, residual shunt after the procedure, different properties of the devices, and antithrombotic treatment remain unclear and make clinical trial design very complex. PFO closure was also proposed in the prevention of decompression illness in divers and to treat POS. Finally, prevention of migraine by PFO closure was investigated in a randomized study without clinical evidence of benefit as compared with medical treatment. The CLOSURE I trial enrolled 909 patients randomized equally to PFO closure using the STARFlex closure device (NMT Medical, Lowell, Mass.) with 24 months of aspirin and 6 months of clopidogrel or to best medical therapy—aspirin, warfarin, or a combination. There were no differences between the two groups with regard to their baseline features. At 2 years, there was no significant difference between the two treatment groups in the rate of recurrent stroke or TIA. Periprocedural major vascular complications occurred in 3.2% of patients in the closure group (13 of 402). Within 6 months, thrombus was found in the left atrium in 1.1% of patients in this group (4 of 366); 2 of the 4 patients with thrombus had a recurrent stroke.42 Bridges and Lock reported the use of the Clamshell septal umbrella (C.R. Bard Inc., Billerica, Mass.) for closure of PFO in 36 patients after a presumed paradoxical embolism during a multicenter clinical trial that was initiated in February 1989 and ended in June 1991.43 Percutaneous PFO closure is now possible with different devices, depending upon availability. The Amplatzer (St. Jude Medical, St. Paul, Minn.) family of devices includes the Amplatzer PFO occluder (Figure 20–4), the multifenestrated (Cribriform) septal occluder (Figure 20–5), and the Amplatzer atrial septal occluder (Figure 20–6). All of these occluders are double-disc devices comprised of nitinol mesh and polyester fabric. Although the atrial septal occluder (ASO, or sometimes known as ASD occluder) was originally designed to close ASDs, it is occasionally implanted in large PFOs. The ASD and PFO occluders are very similar, but there are two important differences between them. The first is that whereas on the ASO the left atrial disc is larger, on the PFO occluder the right atrial disc is larger (except for the 18-mm PFO device, on which both discs are the same size). The other difference is in the diameter of the waist connecting the two discs. In the ASO, the broad connecting waist varies in diameter by device size (up to 40 mm). By contrast, the PFO occluder has a 3-mm connecting waist in each of its three currently available sizes: 18, 25, and 35 mm. The Cribriform device, like the PFO occluder, has a narrow waist, and like the PFO occluder, is available between 18 and 35 mm and is specifically designed to meet multiple needs when occluding multifenestrated ASDs. All the devices can be expanded and collapsed throughout the entire procedure, thereby allowing for complete retrieval up to the point of final detachment from the delivery cable. The PFO occluder is not currently available in the United States.44,45 Figure 20–4 Amplatzer patent foramen ovale (PFO) occluder device. Figure 20–5 Amplatzer Cribriform device. Figure 20–6 Amplatzer atrial septal occluder (ASO). Fischer et al reported on 114 patients with PFO (60 men; age: 47 ± 13 years) and at least one thromboembolic event whose PFO closures were done with the Amplatzer PFO occluder.46 Other causes for embolism were excluded. PFO closure was successful in all patients. After a mean of 10 months, no patient had either a significant residual shunt or a suspected thrombus formation on the occluder. During follow-up study five patients suffered from neurologic events (one stroke, two TIAs, and two epileptic seizures), although complete closure of the PFO was documented by TEE. One patient suffered from bleeding complications (upper gastrointestinal bleeding).46 The CardioSEAL septal occluder (NMT Medical, Boston, Mass.) is no longer produced and was a modified version of the initial clamshell device (Figure 20–7). It consisted of two square Dacron patches, mounted between four spring arms composed of a cobalt-based alloy that bent independently, and were therefore designed to enhance adherence to the interatrial septum. The device was available in diameter sizes from 17 to 40 mm. The STARFlex septal occluder represented a further revision of the CardioSEAL in that it had small microsprings attached at the end of each opposing arm (Figure 20–8). These arms were designed to further enhance positioning in the center of an ASD or PFO. They also provided a closer fit to the septum, thereby decreasing the device profile. To accommodate large-diameter PFOs and ASDs, the STARFlex was also available in a version with six, as opposed to four, arms (device diameter 38 mm), and the system was implanted through a 10F to 12F transseptal sheath. These devices have been discontinued. The prospective FORECAST registry involved 272 patients with presumed paradoxical embolism and PFO who underwent closure with the CardioSEAL and STARFlex devices. The devices were successfully implanted in 99.3% of the patients, with a procedural complication rate of 6.6%. Importantly, stroke or TIA occurred in 1.8% of the patients and device embolization occurred in 0.7%.47 Figure 20–7 CardioSEAL septal occluder. Figure 20–8 STARFlex device. The theoretical advantages of this matrix are particularly relevant with reference to late complications seen with the earlier devices, such as inflammation and thrombus formation.48–50 The BioSTAR device was investigated in the multicenter, prospective, randomized BEST trial evaluating safety and efficacy. The newest development, the BioTREK device, is based on the CardioSEAL BioSTAR technique but is completely resorbable. BioTREK uses a novel bioabsorbable, biosynthetic polymer known as TephaFLEX (Tepha, Lexington, Mass.), a poly-4-hydroxybutyrate (P4HB) that is manufactured by a proprietary biotechnology process utilizing recombinant deoxyribonucleic acid technology. P4HB degrades via a combination of surface erosion and bulk hydrolysis to a natural metabolite (4HB) that is well tolerated and has a short half-life (35 minutes). It is ultimately broken down into carbon dioxide and water via the Krebs cycle, similar to other commonly used bioabsorbable polymers. Similar to BioSTAR, the BioTREK is a platform technology for delivering biological response modifiers. Over time, the patches and the connecting hub disappear, leaving the fibrous septum.51,52 The HELEX septal occluder (WL Gore and Associates, Flagstaff, Ariz.) is a single, super-elastic, spiral-shaped, nitinol wire covered with a biocompatible membrane that is composed of expanded polytetrafluoroethylene (PTFE) (Figure 20–9). After deployment, the nitinol wire forms two flexible, round, equal-sized discs that are fixed by an integral locking system passing through the center of the device from left to right. The wire frame has three eyelets that act as markers and provide good visibility and positioning under fluoroscopic guidance. A locking mechanism connects the right-to-left atrial discs at their respective centers and stabilizes the position of the occluder within the atrial septum. The diameter of the discs ranges from 15 to 35 mm. Figure 20–9 GORE HELEX septal occluder. The HELEX device is implanted through a 9F delivery catheter. This feature spares the use of a long transseptal sheath and likely decreases the risk of air embolism. Advantages of this device are the ease of deployment and the ability to retrieve it throughout the entire procedure and even after release from the delivery catheter via a retrieval cord. In addition, if device embolization occurs after release, retrieval with a snare is easier than with other devices. The new version of this device includes a monorail delivery system and an improved locking mechanism. These two improvements further facilitate delivery and minimize device embolization.53 Ponnuthurai et al reported a study in which 75 adult patients (44.0 ± 11.7 years; 45.3% male) were referred for PFO closure. PFO was found in 69 patients, of whom 68/69 (98.6%) underwent closure with the GORE HELEX device (no PFO was found in five patients, and one patient had an atrial secundum defect closed with the Amplatzer septal occluder). Six of 69 cases required device retrieval, and five of six had successful replacement with a second GORE HELEX device. One of the six had a large PFO associated with atrial septal aneurysm, which was closed with the Amplatzer septal occluder. There were no major complications. At 3-month follow-up, 65/68 (95.6%) had no residual shunt on TTE, and three patients had small residual shunts believed to be related to incomplete endothelialization at 3 months.54 The Premere PFO occluder (St. Jude Medical, St. Paul, Minn.) is specifically designed for PFO closure. The device is composed of two cross-shaped nitinol anchors connected over a flexible polyester braided tether (Figure 20–10). Important features of this device include a variable tether length that can be adjusted according to the thickness of the interatrial septum and tunnel length. Additionally, in an effort to limit foreign material and decrease the device profile, the left atrial side is uncovered, and only the right atrial anchor of the device is covered with a knitted polyester membrane. It is available in diameters of 20 and 25 mm. A 30-mm device is under evaluation. This device is not available in the United States. Rigatelli et al55 reported on 70 patients (48 females and 22 males, mean age 38 ± 6.7 years) with previous stroke who were referred for transcatheter closure of PFO with the Premere occlusion system on the basis of absence of moderate or severe ASA. Forty-six 20-mm and 24 25-mm Premere devices were implanted. Rates of procedural success, predischarge occlusion, and complication were 100%, 95.7%, and 0%, respectively. On mean follow-up time of 40 ± 10.9 months (range 6 to 54), the follow-up occlusion rate was 98.5%. During follow-up study, no cases of permanent atrial fibrillation, aortic/atrial erosion, device thrombosis, or atrioventricular (AV) valve inferences were noted.55 The first use of the Coherex FlatStent PFO occluder (Coherex Medical, Inc., Salt Lake City, Utah) in a human was performed in October 2007 (Figure 20–11). Whereas all other available occluders extend into both atria, this novel device leaves only a minimal amount of surface area exposed in the left atrium. The occluder consists of a super-elastic nitinol lattice with integrated polyurethane foam in the intratunnel cells of the device. This foam is intended to stimulate tissue growth inside the tunnel. The delivery catheter has a monorail design for rapid exchange functionality and is designed to be used by a single operator. The tip of the catheter is shaped to simplify crossing of the PFO. The body of the device expands within the tunnel, drawing the septum primum and the septum secundum into contact without a significant change of the structure of the septum primum. Proximal anchors open into the right atrium, and strategically placed microtines are designed to ensure that the device cannot migrate or embolize. The device can be resheathed and repositioned as necessary until it is detached. Figure 20–11 Coherex FlatStent patent foramen ovale occluder. The device is designed to ensure long-term closure and to have a reduced risk of thrombus formation. There is one size of the FlatStent available for stretched diameters from 4 to 10 mm with a tunnel length of 4 mm or larger. A second device in larger sizes is in development.56 The Cardia family of PFO occluders (Cardia, Burnsville, Minn.) consists of four generations of devices comprised of two Ivalon discs mounted and expanded by nitinol arms (Figure 20–12). The first two generations consist of square discs on four arms, and the second two incorporate hexagonal discs on six nitinol arms. Unique to the current iteration, the Intrasept has a dual articulating sail at the center of the device, which allows for a very low profile against the interatrial septum. The device is available in 5-mm increments from 20 to 35 mm, is delivered through a 9F to 11F transeptal sheath, and is fully retrievable throughout the procedure.57 This device is currently not available in the United States. The Solysafe septal occluder (Swiss Implant, Solothurn, Switzerland) is a self-centering ASD closure device consisting of two synthetic patches that are attached to wires made of a cobalt-based alloy called Phynox (a material similar to nitinol by virtues of elasticity and memory retention) (Figure 20–13). The wires are maintained in place by two wire holders on each side of the atrial septum. A major advantage of this new system is that it is delivered over a guidewire as opposed to a long transseptal sheath. This innovative technique involves the utilization of two control catheters that enable positioning of this device across the PFO and anchoring it against the interatrial septum. Even after complete deployment, the device is fully retrievable up to the point that the guidewire is removed.58 Marketing and distribution of this product ceased on August 2010, in part because of a high rate of wire fracture. The OccluTech device (OccluTech, Jena, Germany) is technically similar to the Amplatzer devices; it is a double-disc device made of a self-expanding nitinol wire mesh (Figure 20–14). Distinguishing it from the Amplatzer devices, there is no hub on the left atrial side. In addition, the Dacron patch on the left side is on the outer surface covering the wire mesh and not inside of the wire mesh.59 The SeptRx (Secant Medical, Perkasie, Penn.) device is a unique device in that it targets only the PFO tunnel as opposed to the right and left atrial borders (Figure 20–15). It is implanted into the flap of the PFO and stretches the defect in an anterior–posterior direction, causing an approximation of septum primum and secundum. Potential advantages of this approach relate to the fact that it does not significantly change the configuration of septum primum and that it minimizes foreign material in the left atrium. This last feature may possibly decrease the risk of thrombus formation.60 HeartStitch (Sutura, Inc., Fountain Valley, Calif.) is a transcatheter polypropylene suture system to close PFOs that is based on the SuperStitch technology (used as a closure technique for femoral vessels) (Figure 20–16). The device consists of individually deployable needles, and its sutures pose no risk of erosion or embolization. The procedure is relatively simple: the HeartStitch system is introduced and deployed into the left atrium. Sequentially, the septum primum and secundum are then sutured and the HeartStitch system is withdrawn. Early investigation is under way.61 The PFx closure system (Cierra Inc., Redwood City, Calif.) (Figure 20–17) is another unique concept in that it is the only non–device-based approach for percutaneous PFO closure. The system is delivered into the overlapping part of septum primum and secundum via the right side of the atrial septum. A vacuum pump functions to hold both septa in place while radiofrequency is applied over an electrode, effectively “welding” the membranes together. The device is then withdrawn from the right atrium, and no foreign material is left behind.62,63 Initial procedure for PFO closure is similar to ASD closure. The timing of the heparin bolus may be delayed if a transseptal needle puncture is needed to gain entry into the left atrium when the PFO is associated with a slitlike tunnel. The use of a sizing balloon during the PFO closure procedure is common, although it is of uncertain value in the opinion of some experts. Balloon sizing is performed by slowly and gently inflating a manufacturer’s sizing balloon in the PFO using a stiff guidewire across the PFO with its distal end in a left pulmonary vein. The waist that appears with balloon inflation can be measured by ultrasound or digital fluoroscopic images with appropriate calibration. The device size is chosen, using the manufacturer’s recommendations, based on the measured diameter of the opened PFO. Finally, the waist may be discrete, which indicates a simple PFO, or more lengthy, which suggests a tunnel-like PFO. The finding of a tunnel PFO leads some operators to perform a transseptal needle puncture to place a more centrally located perpendicular device that overlaps the PFO, rather than using the PFO itself for the location of the center connection of the device, which may be tilted by the tunnel. Other measurements of the patient’s atrial anatomy can be made to estimate where the edge of the device will reach. The additional presence of an ASA often prompts the selection of a larger closure device. Long tunnels represent a particular problem for devices with a fixed distance between the right and left atrial components because there is the potential for both discs to be partially deployed within the tunnel. This can result in a poorly conformed device. Techniques advocated to facilitate successful closure include balloon pull-through, transseptal puncture through the foraminal flap, and delivery of the device though the transseptal puncture site rather than via the tunnel. However, this has the additional risk associated with transseptal puncture, and its success is dependent on precise puncture through the foraminal flap, which can be challenging. It also has a higher residual shunt rate. Another technique that has been described is the balloon pull-through to render the septum primum incompetent and improve the final device position. The balloon pull-through has the disadvantage of being traumatic, and balloon pull-back cannot be fully controlled. Spence et al reported balloon detunnelization, in which a compliant balloon was used to dilate the PFO in a stepwise fashion until the PFO entrance and exit constraint sites approximated and merged on the fluoroscopic image.64 Another method is to use a device such as the Premere occluder for long-tunnel PFOs with an adjustable distance between the left and right atrial discs or a distensible neck, on the basis of its strength and distensible neck rendering it resistant to trapping in long tunnels. Reports on the interventional closure of PFO in combination with ASA showed an increased risk for thrombus formation as well as for incomplete closure. Some operators recommend using a larger device to prevent malposition. Lipomatous hypertrophy of the interatrial septum has a prevalence of 2% and is typically composed of both white and brown fat. The pathognomonic “dumb-bell” shape is caused by hypertrophy of the septum primum and secundum with sparing of the fossa ovalis. A septum secundum more than 15 mm thick has been the predominant defining criterion. Transcatheter closure of a PFO in the setting of lipomatous hypertrophy can be difficult because of the requirement of a device with a long transverse axis. Devices with short waists can result in either inability to capture the septum secundum, leading to incomplete apposition of the device and allowing continued shunt or device embolization. In one case series by Rigatelli et al, eight patients underwent closure via a Premere device and two via the Amplatzer Cribriform device.65 Both patients who underwent closure with the Amplatzer devices continued to have residual shunt resulting from inadequate transverse waist length and malapposition.65 For septa more than 20 mm thick, the Amplatzer muscular ventricular septal defect closure device (AGA Medical, Plymouth, Minn.) has been used to achieve successful closure.66
Patent Foramen Ovale
20.1 Embryology and Anatomy
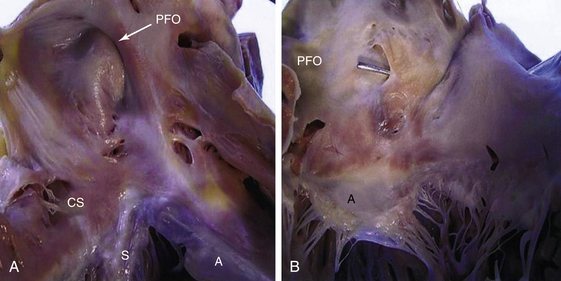
A, Right atrial view of the atrial septum showing the PFO. CS, Coronary sinus; S, septal leaflet of the tricuspid valve; A, anterior leaflet of the tricuspid valve. B, Left atrial view of the atrial septum showing the probe patent PFO. A, Anterior mitral leaflet.
20.2 Incidence
20.3 Associations of Patent Foramen Ovale
Atrial Septal Aneurysm
Chiari Network
20.4 Diagnosis of Patent Foramen Ovale
20.5 Clinical Problems Associated with Patent Foramen Ovale
Patent Foramen Ovale and Stroke
The Platypnea–Orthodeoxia Syndrome
Embolism from Decompression Sickness
Migraine and Vascular Headache
20.6 Indications for Patent Foramen Ovale Closure
20.7 Patent Foramen Ovale Devices
Amplatzer Patent Foramen Ovale Occluder and Septal Occluder
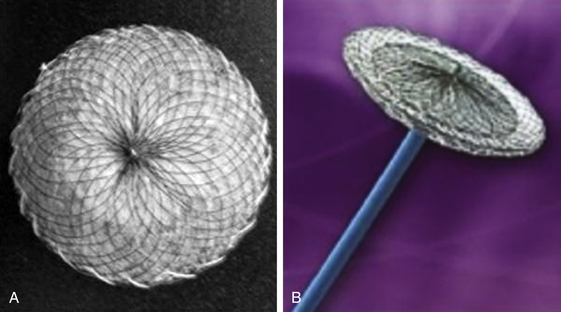
The Amplatzer PFO occluder is a double-disc device comprised of nitinol mesh and polyester fabric with a larger right atrial disc. (Image courtesy St. Jude Medical, St. Paul, Minn.)

The Amplatzer Cribriform device is a double-disc device comprised of nitinol mesh and polyester fabric. (Image courtesy St. Jude Medical, St. Paul, Minn.)
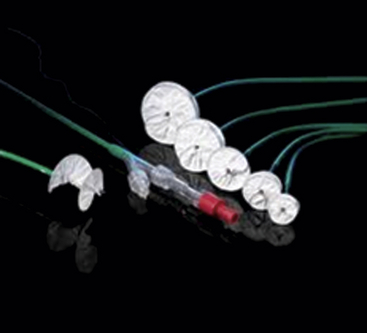
The Amplatzer ASO is a double-disc device comprised of nitinol mesh and polyester fabric with a smaller right atrial disc. (Image courtesy St. Jude Medical, St. Paul, Minn.)
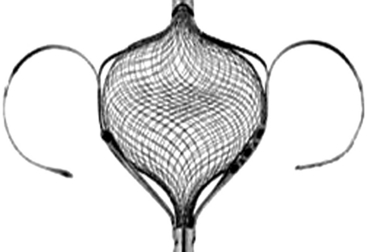
CardioSEAL/STARFlex

The CardioSEAL septal occluder consists of two square Dacron patches mounted between four spring arms. (Image courtesy Nitinol Medical Technologies, Boston, Mass.)

The third-generation STARFlex device has major enhancements with a self-centering mechanism, improved pin-to-pin delivery, and a smaller delivery profile. (Image courtesy Nitinol Medical Technologies, Boston, Mass.)
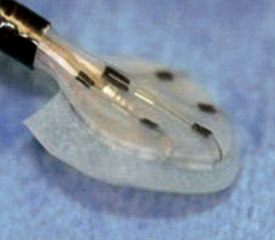
GORE HELEX Septal Occluder
The occluder is comprised of a nitinol wire frame covered with expanded polytetrafluoroethylene. (Image courtesy WL Gore and Associates, Flagstaff, Ariz.)
Premere Patent Foramen Ovale Closure System
The Coherex Flatstent

The occluder consists of a super-elastic nitinol lattice with integrated polyurethane foam in the intratunnel cells of the device. (Image courtesy Coherex Medical, Inc., Salt Lake City, Utah.)
Cardia Ultrasept Patent Foramen Ovale Occluder
Solysafe Septal Occluder
Occlutech
SeptRX Occluder
Heartstitch
PFx
20.8 Implantation Technique
Procedure
Sizing of Patent Foramen Ovale and Selection of Device Size
Patent Foramen Ovale with Long Tunnel
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine