Fig. 25.1
Echocardiography evaluation of duct morphology, in terms of its length and width and its relationship to the descending aortic arch
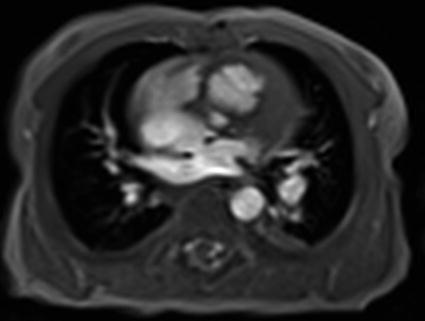
Fig. 25.2
Magnetic resonance imaging (MRI) to analyze pulmonary vein morphology or aortic arch malformations
25.6 Technique (Step-By-Step)
Duct stenting is mostly performed as part of a hybrid procedure during an open chest – beating heart scenario immediately after bilateral PAB [2] or as an elective transcatheter approach in a spontaneously breathing, sedated newborn, with or without an additional atrioseptostomy procedure [1, 3]
The percutaneous transcatheter approach is usually performed by femoral vein or arterial access. In the past, the placement of a 4 F multipurpose catheter was done by passing it through a 4 F sheath (Terumo, Frankfurt, Germany), which was placed in the femoral artery and served for blood pressure monitoring and delineation of the duct-aortic junction. Currently, percutaneous duct stenting is easily performed by femoral artery access utilizing a 4 F delivery system (see Chap. 39).
The step-by-step approach by femoral vein access can be summarized as follows (Fig. 25.3a, b).
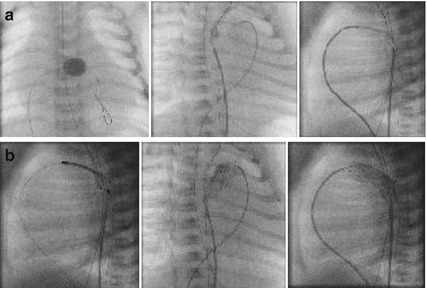





Fig. 25.3
(a) Duct Stenting in Ductus arteriosus dependent Systemic Blood Flow (DA-SBF). How to do it. (b) Duct stenting in DA-SF. How to do it
Step 1 – Evaluation of Interatrial septum/patent foramen ovale (IAS/PFO) (Rashkind +): the interatrial communication must be evaluated. In HLH-S the communication should be unrestrictive or should have no, or only a minimal pressure gradient; in HLH-C patients the interatrial communication should guarantee a sufficient preload for the growing left ventricle.
Step 2, for free crossing of the tricuspid valve a 4 F open-end balloon catheter is used in ensemble with a soft coronary guidewire (BMW, Abbott, Wetzlar, Germany) which is advanced over the duct in the descending aorta (DAO) without the need to follow with a wedge catheter.
Step 3, the balloon catheter is exchanged for a 4 F right Judkins catheter (rJC), which is advanced in the pulmonary artery (PA) and may be advanced over the duct in the DAO.
Step 4, the soft coronary guidewire (BMW) is exchanged for a stiff 0.014 coronary guidewire (S port, Abbott). After pullback pressure measurement from the DAO to the PA is obtained by using a hemostat valve at the Judkins catheter, the rJC is positioned closed to the pulmonary end of the duct. The hemostat valve avoids bleeding and allows angiography by the manual injection of contrast medium. Additionally, a 4 F multipurpose catheter is advanced to the aortic duct junction guided by the BMW coronary guidewire, which can easily be advanced in the aortic arch or through the duct in the pulmonary system; the wire and catheter serve as a marker and the hemostat valve can be used for angiographies from the arterial side performed prior to and during duct stenting.
Step 5, delineation of the duct morphology is performed with biplane angiography in the 30° right anterior oblique (RAO) and 90° lateral planes. This imaging strategy dramatically reduces the interventional time required for percutaneous duct stenting, in addition to improving safety. If the decision for duct stenting is made, the diameter of the stent to be utilized must be at least 1–2 mm greater than the minimal measured duct diameter, but in any case it must exceed the diameter of the DAO. This recommendation is of particular importance in newborns with an interrupted aortic arch.
Step 6, after the preparation of a pre-packed self-expandable Sinus-Superflex-Duct Stent (SSF-DS, OptiMed, Karlsruhe, Germany) and flushing of the 4 F delivery system, the system in total is carefully advanced over the stiff S port wire through the tricuspid valve, the right ventricle, and the PA with the tip advanced to the DAO.
Step 7, the multipurpose catheter together with the BMW guidewire placed in the DAO serves as a marker during expanding of the stent. Slow pulling back of the covering of the delivery system enables full stent expansion to be performed with fluoroscopy control; in some patients short cine scenes are necessary to sufficiently visualize the very thin struts of the open-cell stent.
Step 8, the lateral 90° plane is used to control the stent position at the PA end of the duct (Fig. 25.4).
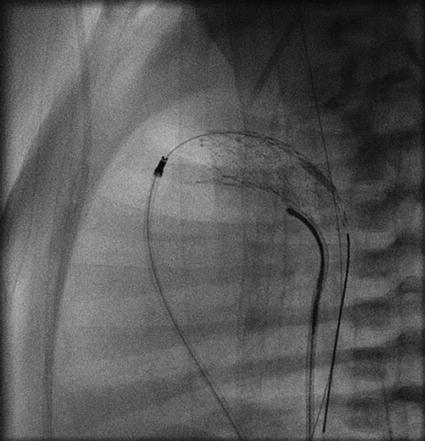
Fig. 25.4
Lateral view showing stent implantation
Step 9, the right anterior oblique (RAO) 30° is the plane of choice to observe the exact stent position in relation to the junction of the duct to the DAO.
Step 10, before the delivery system is carefully removed the stent should have had a short time period during which it has fully expanded with the aid of nitinol conditioning at a temperature of 37° C; then the open-cell device has the chance of expanding within the arterial wall of the duct, and the risk of destabilizing the stent position or inducing inadvertent stent slipping is minimized.
Steps 11 and 12, before the S port wire is carefully removed by re-advancing the rJC to the pulmonary end of the stented duct, the appropriate stent position has to be evaluated by angiographies from the pulmonary and arterial sides, through the Judkins and multipurpose catheters, respectively. The junction of the descending aortic arch to the stented duct, as well as the appropriate positioning of the stent in relation to the pulmonary trunk and left PA has to be carefully defined. It is important that the duct is fully covered by the stent, and the retrograde access to the aortic arch is carefully analyzed.
In most patients there is no need to over-stent the retrograde access to the descending aortic arch; the multipurpose catheter in ensemble with the BMW guidewire easily guides duct stenting and its relation to the descending aortic arch. The indication for the placement of an additional stent within the isthmus is based on pull back pressure measurements and the results of the angiography performed through the multipurpose catheter (see Chap. 39).
Step 13, an additional hemodynamic analysis is recommended after removal of the guiding catheter at the end of the procedure. Noninvasive blood pressure measurement at the right arm, to estimate the coronary and cerebral perfusion pressure, has to be performed simultaneously with invasive pressure measurements of the PA (by the rJC), and DAO pressure measurement by the multipurpose catheter in order to exclude significant pressure gradients across the stented duct and within the aortic isthmus, respectively. The decision for stopping low-dose prostaglandin therapy is based on the hemodynamic and angiographic data obtained; usually the prostaglandin infusion is stopped after the placement of a self-expandable stent, in contrast to the procedure with a balloon-expandable stent, in which the prostaglandin infusion is stopped before heart catheterization to Provoke slight obstruction.< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


