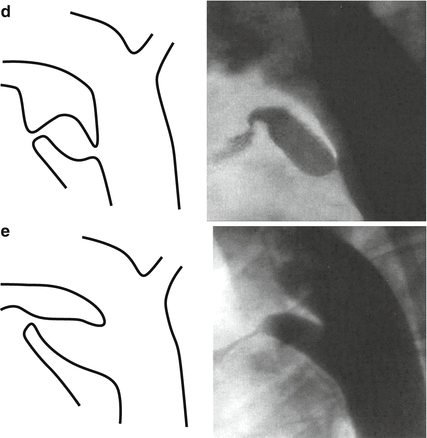
Fig. 29.1
Krichenko classification of PDA morphologies, (a) conical shape, (b) short and wide ductus “Window”, (c) long tubular ductus without constriction, (d) with multiple construction “ complex”, (e) elongated ductus
Different approaches and devices may be needed in different morphologies.
29.2 Indications of Closure and Patient Selection
Clinical scenarios may range from patients with heart failure to asymptomatic subjects. Clinical findings of continuous murmur with bounding pulses and tachycardia indicate significant shunt.
Symptomatic infants who do not respond rapidly to medical anticongestive treatment should undergo PDA closure. Failure to thrive with recurrent lower respiratory tract infections and exertional dyspnea is common in a large PDA with ventricular overload.
Premature neonates commonly have PDA causing morbidity. The standard way of closing PDA in this group is either pharmacological or surgical.
In symptomatic infants weighing less than 5 kg, surgical closure of the PDA is recommended. In fact, the use of devices or coils in small infants with large PDA may have a high incidence of complications. A large device can protrude into the aortic arch causing obstruction. Embolization may occur. The use of large delivery system may induce arrhythmias and can stretch intracardiac structures like the tricuspid valve with severe iatrogenic regurgitation.
In asymptomatic infants with volume-overloaded left heart, PDA closure can be delayed till the weight is above 6–7 kg.
The indication for closure in patients with silent PDA remains controversial. In these patients, the main argument to close the PDA is the prevention of endarteritis.
In adults with PDA, it is possible to have a complete spectrum of disease ranging from small asymptomatic PDA to cases of chronic volume-loaded left heart or to Eisenmenger syndrome.
In patients with markedly increased PVR and those with fixed pulmonary hypertension emphasized by right-to-left shunt (Eisenmenger syndrome), PDA closure is contraindicated. Pulmonary hypertension in this form progresses independently from shunt’s closure.
In patients with moderately increased PVR (>4 Wood units/m2, ratio PVR/SVR > 0.35), the decision is difficult and is based on the pulmonary response to vasoreactivity testing.
Echocardiography is the golden tool to select the cases for transcatheter closure.
29.3 Devices
29.3.1 Coil
In this review, we will describe how to use single Flipper Cook coil to close a small PDA. Other PDA coils (e.g., PFM coil) and the use of multiple coils to close large PDAs are also possible.
Flipper Cook coil comes in different diameters and loop number. They range from 3 mm diameter by 3 loops up to 8 mm diameter by 5 loops. The label in coil package will have 2 numbers (e.g., IMWCE-3-PDA4). The first is the diameter of the loop and second is the number of loops.
The coil is loaded in clear cartridge with wide end at thread of coil side. The delivery system consists of a coil delivery wire (0.035″ thick) with a straightening mandril inside and the wire thread on other side (Figs. 29.2 and 29.3).

Fig. 29.2
Cook coil
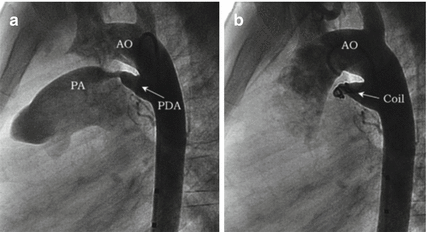
Fig. 29.3
Angiography in lateral view showing a small PDA (a) before and (b) after closure with detachable Cook coil
The Flipper Cook coil is made from 0.035″ wire and can go easily through 4-F end whole only multipurpose catheter that accommodates 0.038″ wire.
29.3.2 Devices
1.
The ADO I device design has a mushroom shape with a low profile and consists of a flat retention disk and a cylindrical main body, into which polyester fibers are sewn. A steel sleeve with a female thread is welded into the marker band at pulmonary end. The retention disk, placed distally at the aortic end, is 4 mm larger than the main body, which itself has a conical structure.
The standard device sizes are 5/4, 6/4, 8/6, 10/8, 12/10, 14/12, and 16/14 mm, respectively.
The first number denotes the diameter of the larger distal (aortic) end of the device at the retention disk whereas the second number, which is always 2 mm smaller, denotes the diameter of the proximal (pulmonary) end where the stainless steel sleeve for screwing onto the cable is located. The smallest first two sizes are 7 mm in length and the remainders are 8 mm.
The delivery system consists of a delivery cable, a Mullins-type sheath, a loader, and a pin vise.
The required delivery sheath sizes from 5 to 8 F.
The size of device chosen is generally such that the diameter of the pulmonary end of the device is at least 2 mm larger than the narrowest diameter of the duct. For example, if the narrowest PDA diameter is 4.8 mm, a 10/8 mm device should be selected.
In adults with large PDA, it is recommended to oversize the device 4 or 6 mm more than the narrowest diameters (Figs. 29.4 and 29.5).
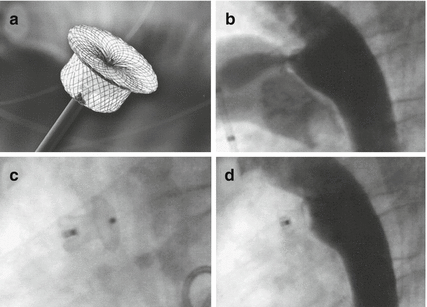
Fig. 29.4
(a) Amplatzer ADO I, (b) angiographic lateral view showing PDA, (c) ADO I in position, (d) aortography in lateral view showing complete PDA closure
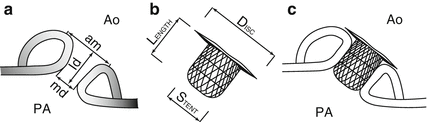
Fig. 29.5
Schematic view of duct measurements and ADO I occluding the PDA
2.

The use of new ADO II and ADO II additional size has proven feasible and effective in providing rapid occlusion of PDAs with a diameter ≥2.0 mm and different morphologies.
Get Clinical Tree app for offline access
The ADO II is a self-expanding nitinol mesh device. Each occluder is made of a multilayered, flexible, fine nitinol wire mesh shaped into a cylindrical waist with retention disks on either end to secure it in the PDA. It has a “fabric-free” technology, which allows for a very low profile of the device and delivery system. The central waist is designed to fill the defect and the two retention disks are designed to be deployed on the arterial and venous sides of the defect.
This design allows deployment from both the arterial and venous sides (Figs. 29.6 and 29.7).
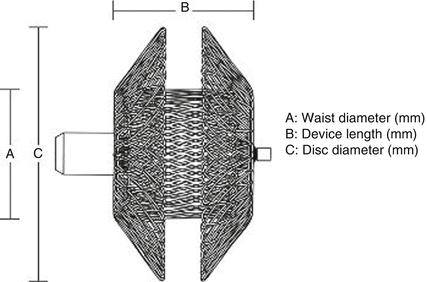
Fig. 29.6
Amplatzer Duct Occluder II. A Waist diameter (mm), B device length (mm), C disk diameter (mm)< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


