2
Passive smoking
Passive smoking is defined as exposure of a (nonsmoking) person to tobacco combustion products from smoking by others. Several synonyms are used in the literature, including involuntary smoking, exposure to environmental tobacco smoke (ETS) and exposure to second-hand smoke (SHS). SHS exposure has been recently recommended as the term to be used, for example by the Tobacco Free Initiative of the World Health Organization [1]. The term ETS was previously used widely, but it seems to have been introduced originally by the tobacco industry and it is recommended to be used less, as it can obscure the preventable nature of this exposure. The term ‘involuntary’ could imply that voluntary smoking would not be as bad for the health, so this term will also be used less in the future.
Passive smoking is still common in homes, workplaces and public places in many countries, although in recent years there has been some progress, with increasing number of countries introducing smoke-free workplace legislation and other tobacco control measures. Some studies have suggested that smoke-free workplaces also reduce smoking at home, and thus lead to reduced passive smoking at home [2]. This may be explained by both increased awareness of the adverse health effects of passive smoking and the reduced active smoking detected in many studies as a consequence of the legislation. However, it is not possible to introduce legislation to protect directly those who are most vulnerable to the harmful effects of SHS exposure at home, i.e. infants, children and the elderly. To protect the health of these susceptible population groups, it is important to increase emphasis on educating people about the adverse effects of passive smoking, and to support smokers to quit or at least to behave in a way that does not expose others to tobacco smoke. In this work, healthcare personnel are among the key players.
This chapter will first introduce definitions related to passive smoking and describe exposure to tobacco smoke, then review the current knowledge on health effects of SHS exposure in children and adults, and finally discuss clinical applications and preventive measures.
In reviewing health effects, assessment of whether the relation between SHS exposure and the health condition is causal is based on the criteria usually used by the recent reviews. These include: (i) the number of studies that have been published on the topic and whether these studies come from different parts of the world; (ii) consistency of findings; (iii) validity of the studies, including control for confounding factors (i.e. other risk factors) and potential biases; (iv) evidence of an exposure-response relation (also called a dose-response relation); (v) evidence of biologically plausible mechanisms; and (vi) evidence of meaningful temporal relation. The best estimate of the effect is given based on recent meta-analyses, which have combined the results of studies published on the health outcome in question. If such a summary estimate is not available, the best effect estimate is given based on a recent, high-quality study.
2.2 Exposure to second-hand smoke
2.2.1 Definitions and constituents of tobacco smoke
Second-hand smoke is composed of sidestream smoke (SS), which is formed from the burning of tobacco products and emitted directly into the environment from the smouldering end of the cigarette between puffs, and exhaled mainstream smoke (MS), which is first inhaled by the smoker before being released into the environment. Other smaller contributors to SHS include smoke that diffuses through the wrapper of the cigarette and smoke that escapes while the smoker inhales. SS is the principal constituent of SHS.
Tobacco smoke is a mixture of thousands of chemicals released into the air as gases, vapors and particles [3]. Over 4000 individual constituents have been identified and these include more than 50 carcinogenic substances as well as many toxic and irritant compounds [4,5]. In addition, several compounds have adverse effects on reproduction. Many constituents are released in higher concentrations in SS than MS because of different burning conditions and less complete combustion of SS. Thus, SS contains higher concentrations of many harmful substances, but is usually then diluted into a larger volume (Table 2.1) [6]. The US National Toxicology Program estimated that at least 250 chemicals in SHS are known to be toxic or carcinogenic. In addition, it is possible that exposure to the mixture of different compounds in SHS is more harmful to health than exposure to any of the individual chemicals, as the compounds may have synergistic effects, i.e. they may have together a larger effect than would be expected from summing up the effects of individual compounds [7]. There is some evidence suggesting that evaporation of biologically less active components may cause aged sidestream smoke to be more toxic on a weight-for-weight basis.
Table 2.1 Emissions of selected tobacco smoke constituents in fresh, undiluted mainstream smoke (MS) and diluted sidestream smoke (SS) from nonfiltered cigarettes [6]
| Constituent | Amount in MS per cigarette | SS:MS ratio |
| Established carcinogensa | ||
| Benzene | 12-48 μg | 5-10 |
| 2-Naphthylamine | 1.7 ng | 30 |
| 4-Aminobiphenyl | 4.6 ng | 2-4 |
| Nickel | 20-80 ng | 13-30 |
| Toxic or irritant | ||
| Carbon monoxide | 12-23 mg | 2.5-4.7 |
| Formaldehyde | 70-100 μg | 0.1-50 |
| Acrolein | 60-100 μg | 8-15 |
| Nitrogen oxides | 100-600 μg | 4-10 |
aIARC category 1 = carcinogenic to humans.
SHS exposure usually means passive smoking by nonsmokers. However, smokers are exposed to particularly high concentrations of sidestream smoke, because their own smoking is the major source of it and because they spend more time in smoky environments. Thus, SS may contribute to the adverse health effects detected in active smokers, but as this has not been studied much, this chapter will focus on the health effects of passive smoking in nonsmoking populations, which have been studied extensively. It should be noted that a fetus can be exposed to tobacco smoke by either the mother’s active smoking during pregnancy or a nonsmoking mother’s exposure to SHS. Both of these influence the development of the fetus, as tobacco smoke constituents are transferred across the placenta, so both of them result in fetal passive smoking. This chapter will focus on fetal passive smoking from the mother’s SHS exposure during pregnancy.
For young children, smoking adults at home, especially the parents, form the principal source of SHS exposure. With increasing age, other places contribute as sources of SHS exposure: first day-care facilities and then school and many social environments. Among adults, home and workplace are the major sources of SHS exposure, because of the long time periods usually spent in these environments. However, some social environments, such as bars, restaurants and public transport, have been found to have particularly high concentrations of SHS. This chapter will focus on SHS exposure at home. It will briefly also mention SHS exposure at work, but other chapters will discuss SHS exposure in other environments.
2.2.3 Occurrence of SHS exposure
The prevalence of SHS exposure varies considerably between countries and is influenced by the prevalence of active smoking, the traditions and behavioral cultures, the tobacco control legislation and the healthcare and educational systems. Multicenter studies from North America and Europe have measured cotinine in body fluids as an indicator of passive smoking and found that, in the 1980s, more than 80% of the nonsmoking populations were exposed to SHS. They also showed an alarming trend for the highest exposures to be detected in children and young adults. Today there is more variability in SHS exposure within Europe and between different states of the USA, as some countries and states have adopted smoke-free workplace and other forms of stricter tobacco control legislation, while others have not yet taken these preventive steps. For example, estimates of the prevalence of passive smoking of children from Europe have ranged from 7-15% in Finland and Sweden to 70-75% in Bulgaria and Poland. SHS still remains the most important preventable indoor exposure even in many high-income countries. The smoking epidemic in low-income countries seems unfortunately to continue, meaning that a high proportion of children in such countries are exposed to SHS. These children may be especially vulnerable to the harmful effects of SHS, as they may suffer also from malnutrition and may be exposed to other harmful compounds, for example from use of solid fuels that may act synergistically with SHS. WHO has databases on smoking prevalences and tobacco control legislations across the world (http://www.who.int/tobacco/global_data/en/index.xhtml).
2.2.4 Measuring exposure to SHS
Exposure to SHS can be assessed using different methods depending on the purposes of the measurements [7]. The most direct method to measure SHS exposure is to use personal monitors available for individual tobacco smoke components, such as nicotine or respirable suspended particles (RSP). However, this method requires a lot of labor, is rather expensive and only measures current exposure for a short interval. Individual tobacco smoke components can also be measured by fixed monitors in defined spaces. When combining the results of such measurements with information on time-activity patterns, an individual’s or a population’s exposure to SHS can be assessed. Again, this method only measures current exposure for a rather short interval, is expensive, and only measures exposure to specific compounds rather than to the entire mixture. However, such measurements may be useful, for example, when assessing the effectiveness of smoke-free workplace policy.
Studies of health effects have most commonly applied questionnaires or diaries to assess SHS exposure. These methods have the advantages of being cheap and providing the possibility to measure long-term exposure which may be more relevant for many health effects [8]. Questionnaires can also inquire into past exposures. This is the relevant exposure, for example, when investigating lung cancer, as the relevant exposure has taken place at least 10 years earlier because of the long lag time. A potential problem related to questionnaires and diaries is whether people remember and report their exposures correctly. Many studies that have compared questionnaires with other exposure assessment methods suggest that questionnaires provide valid information, i.e. the majority of people report correctly whether they have been or have not been exposed to SHS, but that the exact quantification of exposure may not be very precise. However, it is still likely that people are able to recall rather well whether they have been exposed heavily or lightly.
Another way to assess exposure to SHS is to measure biomarkers, i.e. compounds, their metabolites, hemoglobin or DNA adducts in biological samples, which are influenced by the uptake, metabolism and elimination mechanisms in addition to the exposure concentration. These may give relevant information about exposure to some target organs. The most commonly measured biomarker of tobacco smoke is cotinine in serum, saliva or urine. Cotinine is a major metabolite of nicotine. Its half-life is about 20 h, so it measures only recent exposure over the last 1-3 days. As a consequence of this, it may not be good assessment method for diseases for which long-term exposure is relevant. Hair nicotine concentration has been measured in some recent studies and seems to reflect exposure over the last 2 months. Some studies have also measured biomarkers of the carcinogenic substances, for example amino biphenyl hemoglobin adduct. Biomarkers are indicators for total exposure across different microenvironments, including home, workplace and social settings. For health effect studies, it has been recommended to use a combination of a questionnaire and some other method, if there are enough resources available.
2.3 Health effects of passive smoking in children
Children are more susceptible to the adverse effects of SHS than adults for several reasons. Their respiratory system is not fully mature at birth and continues to develop both immunologically and physiologically. Children have higher breathing rate and inhale more air per body volume than adults, which results in higher exposure with a similar SHS concentration. In addition, children’s liver metabolism and other clearing mechanisms are not yet fully developed, so the harmful substances remain longer in the body. Some studies have suggested that children who were exposed to tobacco smoke in utero through either active or passive smoking by the pregnant mother are at greater risk for developing SHS-related diseases later, so tobacco smoke exposure in the very early phases of lung development may also make children more vulnerable later in life.
This section will first discuss health effects related to SHS exposure from the mother’s passive smoking during pregnancy and then health effects related to the child’s passive smoking after birth. However, these exposures are highly correlated, as is maternal smoking during pregnancy and the child’s postnatal SHS exposure, so it has not been easy to disentangle the effects of these exposures.
2.3.1 Health effects of mother’s passive smoking during pregnancy
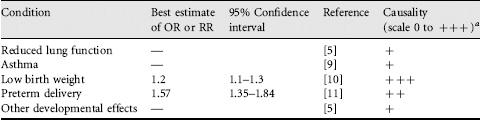
Health effects related to mothers’ SHS exposure during pregnancy are summarized in Table 2.2.
Table 2.2 Summary of health effects of mothers’ passive smoking during pregnancy
a0 = no evidence of a relation between passive smoking and this condition; + = some evidence of a relation between passive smoking and condition; + + = strong but not definitive evidence of a causal relation between passive smoking and condition; + + + = established causal relation between passive smoking and condition.
Lung function impairment
Maternal smoking during pregnancy has been linked to reduced lung function in infants in many studies. According to recent reviews [5,9], there is also evidence of adverse effects of maternal passive smoking during pregnancy on the child’s lung function. However, as the number of studies looking at this question is limited, no definite conclusions concerning effects of mother’s SHS exposure during pregnancy on child’s lung function can be made.
Asthma
Maternal smoking during pregnancy has been strongly linked to the risk of childhood asthma [12], but again, the overall number of studies looking at the effects related to mother’s SHS exposure during pregnancy is limited [9].
Low birth weight
Active smoking by the mother is a well-known cause of low birth weight (LBW). There is increasing literature also on nonsmoking mothers’ exposure to SHS and low birth weight [9,13]. Low birth weight has usually been defined as birth weight <2500 g either preterm or at full term (≥ 37 weeks of gestation) birth. When LBW occurs at full term, it means that the fetal growth was reduced and the outcome is called small for gestational age (SGA). A review of this topic by US Surgeon General in 2006 [5] identified 43 cohort and three case-control studies on LBW or SGA. The most recent meta-analysis included 19 studies and gave a summary risk ratio of 1.2 (95% confidence interval, CI, 1.1-1.3), meaning a 20% excess risk in children of exposed mothers [10]. The average effect on birth weight was estimated as − 28 g (− 41 to − 16) in exposed infants compared with unexposed infants. The overall judgment based on recent reviews is that mothers’ passive smoking is causally linked to low birth weight of the infant [5,9,11,13]. This causal effect seems to be a consequence of reduced oxygen in the fetus, which is attributable to CO exposure from SHS and nicotine-induced vasoconstriction, leading to reduced blood flow of uterus, placenta and umbilical cord.
Preterm delivery and other developmental effects
The other pregnancy outcome that has been linked to mother’s passive smoking is preterm delivery, defined as <37 completed weeks of gestation [13]. The strongest evidence comes from a population-based Finnish study that found an OR of 1.30 (95% CI 0.30-5.58) for SHS exposure in the middle range and 6.12 (1.31-28.7) for the highest SHS exposure based on nonsmoking mother’s hair nicotine concentration [14]. The recent meta-analysis by California Environmental Protection Agency [11] gave a summary relative risk of 1.57 (1.35-1.84) for preterm delivery, meaning 57% excess risk in children of exposed mothers. However, not all studies have found consistent results, so more studies are needed before definite conclusions on causality of preterm delivery can be made.
Other developmental effects that have been linked to mothers’ passive smoking include spontaneous abortion and perinatal death, congenital malformations and impaired neuropsychological and physical development, but because of limited evidence, no definite conclusions can be made concerning the strength of these relations [5]. Maternal SHS exposure has also been linked to increased persistent pulmonary hypertension of the newborn.
2.3.2 Health effects of passive smoking in childhood
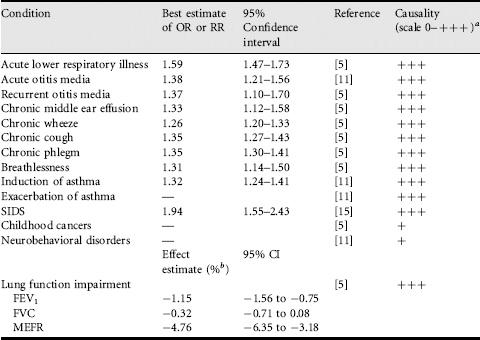
The first studies reporting a link between parental smoking and respiratory disease in children were published in the early 1970s. Since then abundant evidence on adverse health effects of SHS exposure in childhood has accumulated. This is summarized in Table 2.3.
Table 2.3 Summary of health effects of passive smoking in childhood
a0 = no evidence of a relation between passive smoking and condition; + = some evidence of a relation between passive smoking and condition; + + = strong but not definitive evidence of a causal relation between passive smoking and condition; + + + = established causal relation between passive smoking and condition.
bPercentage difference of lung function in children exposed to SHS compared with unexposed children.
Acute lower respiratory illnesses
More than 100 studies from different parts of the world have been published on parental smoking in infancy and early childhood and the risk of the child’s acute lower respiratory illness. These have consistently shown an increased risk of acute lower respiratory illnesses, including respiratory infections such as acute bronchitis, bronchiolitis, respiratory syncytial virus infections and pneumonia, and in some studies also symptoms of the lower respiratory tract [5,9,11]. There is evidence of an exposure-response relation, meaning that the risk of the disease increases with increasing amount of exposure, measured as the number of smoking parents and other household members or the number of cigarettes smoked at home. The most recent meta-analysis of these studies was conducted by the US Surgeon General [5] and gave a summary odds ratio of 1.59 (95% CI 1.47-1.73), suggesting an excess risk of 59% among children exposed to parental smoking.
The risk related to mother’s smoking is higher (OR 1.72, 1.59-1.86) than that related to father’s smoking (OR 1.31, 1.19-1.43), but both maternal and paternal smoking increase significantly the child’s risk of getting lower respiratory illness. The higher risk from mother’s smoking could be explained by small children usually spending more time with their mother than with other adults, or by a synergistic effect between childhood maternal smoking and maternal smoking during pregnancy, as these often correlate. The risk from parental smoking seems to be highest in young children. For example in a meta-analysis by Li and co-workers [16], the summary RR was 1.71 (1.33-2.20) in children 0-2 years old. The smaller effect in older children has been explained by less time being spent in the presence of household smokers with increasing age as well as by maturation of the immune system of the child.
In terms of biologically plausible mechanisms for the relation between passive smoking and lower respiratory illness, tobacco smoke is known to impair the immunological defense mechanisms as well as the function of airway cilia, both of which are likely to lead to increased susceptibility to infections. In addition, SHS has been shown to enhance bacterial adherence and disrupt respiratory epithelium, which is an important host defense barrier. In conclusion, all recent reviews have concluded that parental smoking is causally linked to increased acute lower respiratory illnesses, especially in young children [5,9,11].
Otitis media
According to US Surgeon General’s Report in 2006 [5], 59 studies from different parts of the world have investigated the relation of parental smoking to middle ear disease in children. A causal association has been found with acute and recurrent otitis media as well as chronic middle ear effusion. The best estimates of relative risks from recent reviews are 1.38 (1.21-1.56) for acute otitis media and 1.37 (1.10-1.70) for recurrent otitis media in relation to either parent smoking, meaning 30-70% excess risk. The relative risk of chronic middle ear effusion is 1.33 (1.12-1.58). Potential mechanisms that underlie these relations include decreased mucociliary clearance leading to increased susceptibility to infections and Eustachian tube dysfunction due to mucosal swelling that can lead to accumulation of effusion in the middle ear [11].
Chronic respiratory symptoms
Since the first studies on parental smoking and chronic respiratory symptoms in children were published in the early 1970s, a large number of studies on this topic from different parts of the world have been reported. The recent report by the US Surgeon General [5] included 88 studies in their quantitative overview. The summary relative risks related to either of the parents smoking were 1.26 (95% CI 1.20-1.33) for wheeze, 1.35 (1.27-1.43) for cough, 1.35 (1.30-1.41) for phlegm and 1.31 (1.14-1.50) for breathlessness, meaning 26-35% excess risk in children of smoking parents. All symptoms showed increasing risk with increasing number of parents smoking at home, suggesting exposure-response relation. Generally the risk was higher in relation to mother’s smoking, but father’s smoking was also related to significantly increased risk. All recent reviews have concluded that parental smoking is causally related to chronic respiratory symptoms in children [5,9,11]. Tobacco smoke contains many substances that can induce irritation and chronic inflammation in the airways, and these mechanisms are likely to underlie the observed relations with respiratory symptoms. Wheeze is a symptom of both respiratory infections and asthma in children, and so reflects disease mechanisms of these conditions, as reviewed separately.
Asthma
Induction of asthma. About 85 studies from different parts of the world have addressed the risk of developing asthma in childhood in relation to parents’ smoking. The most updated meta-analysis of these was conducted by the California Environmental Protection Agency [11] in 2005. Its meta-analysis was based on 29 studies and gave a summary RR for new-onset asthma of 1.32 (95% CI 1.24-1.41), meaning 32% excess risk in children whose parent(s) smoke. The risk was higher in preschool children (1.44, 1.04-1.99), but remained significantly increased also in older children. When the child was exposed to parental smoking both during pregnancy and after birth the risk was strongest, but significant increase in the risk was detected also in association with postnatal SHS exposure only. The risk of asthma increased with increasing duration of passive smoking, suggesting an exposure-response relation: RR was 1.22 (1.16-1.34) for 5 years of postnatal SHS exposure and 1.42 (1.28-1.70) for 10 years of such exposure. In addition to mechanisms that will be discussed in connection with adult asthma, in infants other mechanisms may play a role. These include impaired airway development during pregnancy and in infancy in those exposed to passive smoking and the influence of SHS on development of immunological responses, for example the balance between Th1 and Th2 cells [5].
Exacerbation of asthma. Several studies have shown that parental smoking is a causal factor for exacerbation of asthma in children with a pre-existing disease [11], in addition to increasing the risk of new asthma in previously healthy children. Different types of outcomes related to exacerbation of asthma have been studied, including the frequency and severity of asthma symptoms, use of asthma medications, school absenteeism, use of healthcare services, hospitalization and changes in lung function parameters, such as peak expiratory flow (PEF). In longitudinal studies, the effects of passive smoking have been detected most consistently on increased asthmatic symptoms, more and prolonged use of medication, and increased school absenteeism.
Lung function impairment
There are numerous cross-sectional and some longitudinal studies showing that parental smoking is linked to lung function deficits as well as to reduced growth of lung function in children. As discussed above, exposure during pregnancy seems to be of importance, but postnatal SHS exposure has also been shown to have significant adverse effect on lung function of children. The recent review by US Surgeon General [5] included 26 studies and measured the summary effect as percentage differences of lung function in children exposed to SHS compared with unexposed children. The effects were −1.15% (95% CI − 1.56 to − 0.75) on forced expiratory volume in 1 second (FEV1), −0.32% (−0.71 to −0.08) on forced vital capacity (FVC), and −4.76% (−6.34 to −3.18) on mid-expiratory flow rate (MEFR).
Overall the results show small but significant adverse effect of childhood passive smoking on spirometric lung function, which is likely to persist into older ages. This effect has been judged by most recent reviews to be causal [5,11]. In addition, there is some evidence that passive smoking may lead to reduced diffusing capacity of the lungs [11].
Sudden infant death syndrome
Sudden infant death syndrome (SIDS) is a sudden, unexpected and unexplained death of an infant before one year of age. A review from 1997 identified 39 studies that had investigated the risk of SIDS in relation to passive smoking after birth and gave a summary relative risk of 1.94 (95% CI 1.55-2.43), meaning 94% excess risk in infants exposed to SHS [15]. Most studies assessed exposure from mother’s smoking after birth and one-third of them controlled for maternal smoking during pregnancy (i.e. prenatal exposure). Also father’s smoking has been significantly linked to increased risk of SIDS. Several studies have shown evidence of exposure-response relation with the amount of SHS exposure. All recent reviews have concluded that there is a causal relation between parental smoking and SIDS [5,11]. Exposure to nicotine and toxicants in tobacco smoke has been shown to have neurotoxic effects, affecting neuroregulation of breathing and apnoeic spells. SHS exposure has been found to be associated with a change in the ventilatory and cardiac responses to hypoxia [5,11].
Childhood cancers
Childhood cancers are relatively rare conditions. One cohort and some case-control studies have investigated the relations of childhood cancers to parental smoking. The strongest evidence links maternal smoking to overall childhood cancer risk. Of specific cancers, SHS exposure has been associated with leukemias, lymphomas and brain tumors. Few studies have distinguished the effects of postnatal exposure from exposure during pregnancy. Relevant exposure may have occurred already before conception, i.e. through mutations of male germ cells. In view of the rather small number of studies adjusting for other potentially important cancer risk factors, the relations between SHS exposure and childhood cancers have not been judged as causal and more studies on this topic are needed [5].
Neurobehavioral and other effects
There is some evidence that children’s cognition and behavior are adversely affected by passive smoking [5,11]. A large study based on the third US National Health and Nutrition Examination Survey (NHANES III) showed a significant inverse relation between child’s serum cotinine level and performance on cognitive tests: decrements in cognitive scores were detected at higher cotinine levels, i.e. among those with more exposure to SHS. A large British study showed that children whose mother smoked had lower scores in a vocabulary test. However, not all studies have found such effects and more studies are needed before any definite conclusions can be made.
Children’s passive smoking has also been linked in recent studies to significantly increased caries in young children and less favorable serum lipid profile in children up to 15 years of age, including significantly lower levels of high-density lipoprotein cholesterol (HDL).
2.4 Health effects of passive smoking in adults
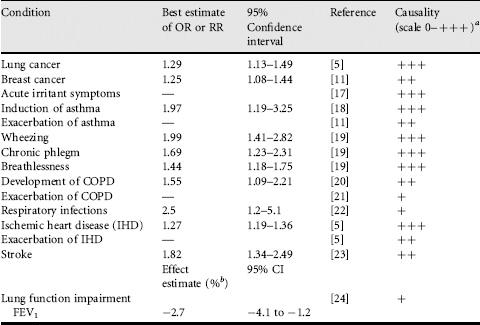
The first studies linking passive smoking to adverse health effects in adults were from the early 1980s and focused mainly on lung cancer. More recently there has been increasing research also into nonmalignant effects of SHS exposure in adulthood. The evidence from adult studies is summarized in Table 2.4.
Table 2.4 Summary of health effects of passive smoking in adulthood
a0 = no evidence of a relation between passive smoking and condition; + = some evidence of a relation between passive smoking and condition; + + = strong but not definitive evidence of a causal relation between passive smoking and condition; + + + = established causal relation between passive smoking and condition.
bPercentage difference of lung function in adults exposed to SHS compared with unexposed adults.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


