Little is known about the familial aggregation of intermittent claudication (IC). Our objective was to examine whether parental IC increased the risk of IC in adult offspring, independent of the established cardiovascular risk factors. We evaluated the Offspring Cohort Participants of the Framingham Heart Study who were ≥30 years old, cardiovascular disease free, and had both parents enrolled in the Framingham Heart Study (n = 2,970 unique participants, 53% women). Pooled proportional hazards regression analysis was used to examine whether the 12-year risk of incident IC in offspring participants was associated with parental IC, adjusting for age, gender, diabetes, smoking, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, and antihypertensive and lipid treatment. Of the 909 person-examinations in the parental IC history group and 5,397 person-examinations in the no-parental IC history group, there were 101 incident IC events (29 with parental IC history and 72 without a parental IC history) during follow-up. The age- and gender-adjusted 12-year cumulative incidence rate per 1,000 person-years was 5.08 (95% confidence interval [CI] 2.74 to 7.33) and 2.34 (95% CI 1.46 to 3.19) in participants with and without a parental IC history. A parental history of IC significantly increased the risk of incident IC in the offspring (multivariable adjusted hazard ratio 1.81, 95% CI 1.14 to 2.88). The hazard ratio was unchanged, with an adjustment for the occurrence of cardiovascular disease (hazard ratio 1.83, 95% CI 1.15 to 2.91). In conclusion, IC in parents increases the risk of IC in adult offspring, independent of the established risk factors. These data suggest a genetic component of peripheral artery disease and support future research into genetic causes.
A National Institutes of Health consensus statement has described family history as vital to patient care, because it can uncover information about factors that contribute to the risk of developing common diseases, such as diabetes mellitus, stroke, cancer, and heart disease. The occurrence of cardiovascular disease (CVD) in a parent or sibling confers an increased risk of CVD in middle-age adults, distinct from the traditional risk factors. The parental occurrence of stroke is associated with a threefold increase in the risk of offspring stroke. However, little is known about the familial aggregation of peripheral artery disease (PAD). The subjects whose siblings were diagnosed with premature PAD were shown to have an almost threefold increase in PAD. Finally, the early onset of symptomatic CVD is more common in first-degree relatives of patients with premature PAD than in the relatives of healthy persons. The latter 2 studies were limited by the small sample size, an examination of PAD prevalence rather than incidence, and an inability to quantify the degree of familial aggregation of PAD that was independent of the established risk factors. The Framingham Heart Study (FHS) affords the unique opportunity to study intermittent claudication (IC) across 2 generations using prospectively collected data in a large community-based sample. The present study was undertaken to test the hypothesis that parental IC confers an increased risk of IC in adult offspring, independent of established CVD risk factors.
Methods
The FHS is a prospective epidemiologic cohort study that was established in 1948 when 5,209 residents of Framingham, Massachusetts, aged 28 to 62 years, were enrolled. The members of the original cohort have undergone examinations every 2 years. In 1971, the offspring of the original cohort (n = 3,548) and the spouses of the offspring (n = 1,576), aged 5 to 70 years, were enrolled into the Framingham Offspring Study. The offspring cohort has undergone an examination about every 4 to 8 years. All participants provided informed consent, and the institutional review board of Boston University School of Medicine approved all study protocols.
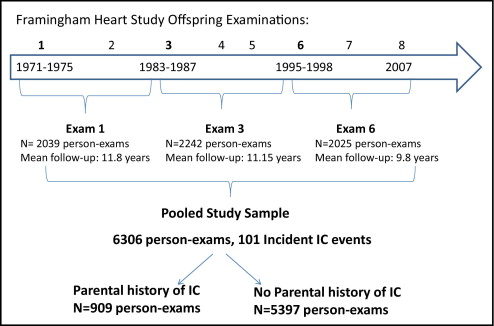
The data from 3 offspring examinations ( Figure 1 ) , each with 12 years of follow-up, were pooled: offspring examination 1 (1971 to 1975), 3 (1983 to 1987), and 6 (1995 to 1998). The follow-up for the final examination ended in December 2007. Given the structure of the follow-up examinations in the offspring, we chose to study the 12-year incidence of IC to have a comparable length of nonoverlapping follow-up after each baseline examination. All offspring participants who were ≥30 years old at any of the 3 baseline examinations were eligible if both parents were enrolled in the original cohort and if the Offspring participant was free of CVD and IC at the examination. A total of 2,970 unique subjects (1,405 men) were included in our final study sample, including 346 with a parental history of IC and 2,624 with both parents free of IC. Parental IC was defined as the occurrence of IC in a parent before the offspring examination. Both parental IC events and incident IC events occurring in the offspring were adjudicated by a panel of 3 senior investigators using the same previously established criteria. The investigators were unaware of the parental IC status. All available information was used to determine the presence of IC, including the standardized physician-administered IC questionnaire that was a part of each routine FHS research clinic visit, any available records from the office visits with the participants’ personal healthcare provider, and the hospital records pertaining to PAD. The standardized physician-administered questionnaire asked about the presence of calf and leg discomfort brought on by exertion, the relation of the discomfort to the rapidity of walking or uphill walking, and whether the symptoms were relieved with rest. The final diagnosis of IC was determined from the clinical history only, without confirmatory testing. At the more contemporary FHS examinations, the participants were queried about lower extremity revascularization procedures and all self-reports were validated from the medical records.
At each offspring examination, the risk factors were directly measured and the occurrence of CVD was updated. Blood pressure at rest was measured twice. Current smoking was defined as smoking ≥1 cigarettes/day in the year preceding the examination. Blood was drawn, with the patient in the fasting state, for total cholesterol, high-density lipoprotein cholesterol, and triglyceride measurements. Diabetes was defined as a fasting glucose level of ≥126 mg/dl (7.0 mmol/L) or the use of insulin or oral hypoglycemic agents. CVD was defined as any of the following events: myocardial infarction, coronary insufficiency, angina pectoris, stroke, transient ischemic attack, congestive heart failure, or cardiovascular death.
The follow-up time within each 12-year period was calculated as the interval from each baseline visit date until the diagnosis date of IC for those participants who developed the disease and censored at the earliest of the date of the last examination, date of death, or end of the 12-year period for participants who did not develop the disease. The age- and gender-adjusted incidence rates and 95% confidence intervals (CIs) per 1,000 person-years were calculated in each parental IC history group by dividing the number of IC events observed by the total person-years. The Kaplan-Meier curves and the log-rank test were used to plot and compare the cumulative incidence rates. Pooled proportional hazards regression analyses were used to examine whether the 12-year risk of incident IC in offspring was associated with parental IC. This method of pooling person-examinations provides estimates of the effect similar to a time-dependent Cox proportional hazards model. Furthermore, this method allowed us to update the risk factors and parental IC at each examination. The hazard ratios (HRs) and 95% CIs were calculated with the reference group consisting of participants with no parental IC before the examination. The covariates used in the multivariable model included age, gender, diabetes, current smoking, systolic blood pressure, antihypertensive treatment, total cholesterol, high-density lipoprotein cholesterol, and cholesterol lowering treatment. In the secondary analyses, we adjusted for the occurrence of CVD in the Offspring participants using 2 approaches. First, we used Cox models in which CVD was entered as a time-dependent covariate. Next, the follow-up time was censored when an Offspring participant developed any CVD event to account for the fact that CVD increases the risk of IC. To assess the incremental predictive utility of parental IC history associated with incident IC in the offspring, we calculated the c-statistic for the model with clinical covariates alone and the full model with clinical covariates and parental IC history. We assessed the model calibration (i.e., concordance of observed risk and that predicted by the model with parental IC history) by calculating the Hosmer-Lemeshow Chi-squared statistic for the Cox models. To evaluate whether the inclusion of parental IC history improved the risk classification of participants, we calculated the enhanced “net reclassification improvement” (NRI) using an extension to survival analysis that uses Kaplan-Meier estimates of event probabilities at 12 years. We used 12-year IC risk thresholds of <2%, 2% to 5%, and >5% for the NRI index. The NRI is used to assess how well a new marker “reclassifies” patients from 1 risk category to another. Because no categories for the absolute risk of IC have been previously established, we also assessed the “category-less” NRI, which assesses any upward or downward reclassification. Values >0 correspond to improved reclassification. We performed a secondary analysis defining the incident events in the Offspring as IC and or lower extremity revascularization. All statistical analyses were performed using SAS statistical software, version 9 (SAS Institute, Cary, North Carolina). Statistical significance was defined as a 2-tailed p value <0.05.
Results
The baseline characteristics of the offspring study sample are listed in Table 1 . The group of participants with parental history of IC was older (mean age 49.9 vs 47.6 years, p <0.0001) and, with the exception of current smoking, had significantly greater risk factor levels compared to the group of participants without a parental history of IC. During the follow-up period, 101 incident IC events (29 in participants with a parental history of IC and 72 in participants with no parental history of IC) occurred. The age- and gender-adjusted 12-year cumulative incidence rate per 1,000 person-years was 5.08 (95% CI 2.74 to 7.33) in participants with a parental history of IC and 2.34 (95% CI 1.46 to 3.19) in participants with no parental history of IC (log-rank test, p <0.0001; Figure 2 ) . Parental IC was associated with a significantly increased risk of incident IC in offspring (age- and gender-adjusted HR 2.29). The association was modestly attenuated but remained significant after adjustment for traditional risk factors (multivariable-adjusted HR 1.81, 95% CI 1.14 to 2.88; Table 2 ). The association was unchanged after additional adjustment for interim CVD and the magnitude and significance of the effect of parental IC persisted in the analysis in which the follow-up time was censored when the offspring participant developed an incident CVD event ( Table 2 ). The addition of parental IC history to a multivariable model incorporating the baseline covariates increased the already high c-statistic from 0.831 (95% CI 0.794 to 0.868) to 0.837 (95% CI 0.801 to 0.873). The 2 c-statistics were not significantly different statistically (p = 0.22). The model with parental IC history had excellent calibration (Hosmer-Lemeshow Chi-squared 14.07; p = 0.20). The category-based NRI was modest (9.3%, 95% CI 1.9% to 17.3%) and the category-free NRI was 34.5% (95% CI 15.5% to 55.6%). The NRI estimates remained essentially unchanged, with adjustment for the occurrence of CVD entered as a time-dependent covariate or in a model in which the follow-up time was censored when a participant developed any CVD event. The association between parental IC and incident PAD, defined as IC and/or lower extremity revascularization (n = 114 events), was very similar to the primary analysis (multivariable-adjusted HR 1.76, 95% CI 1.14 to 2.72; additional adjustment for CVD as a time-dependent covariate [HR 1.77, 95% CI 1.15 to 2.73] and censoring at the first occurrence of CVD [HR 1.84, 95% CI 1.13 to 2.97] did not substantively change the association).
| Variable | Parental History of IC ⁎ | p Value | |
|---|---|---|---|
| No (n = 5,397 Person-Examinations) | Yes (n = 909 Person-Examinations) | ||
| Age (years) | 47.6 ± 11 | 49.9 ± 10.5 | <0.0001 |
| Women | 2,927 (54%) | 466 (51%) | 0.10 |
| Current smoker | 1,501 (28%) | 276 (30%) | 0.12 |
| Diabetes mellitus | 202 (4%) | 62 (7%) | <0.0001 |
| Systolic blood pressure (mm Hg) | 123 ± 17 | 126 ± 17 | <0.0001 |
| Diastolic blood pressure (mm Hg) | 78 ± 10 | 79 ± 10 | 0.003 |
| Total cholesterol (mg/dl) | 206 ± 40 | 210 ± 41 | 0.005 |
| High-density lipoprotein cholesterol (mg/dl) | 52 ± 15 | 50 ± 16 | 0.007 |
| Triglycerides (mg/dl) | 119 ± 126 | 131 ± 105 | 0.007 |
| Lipid-lowering medications | 139 (3.6%) | 42 (4.6%) | 0.001 |
| Body mass index (kg/m 2 ) | 26 ± 4.8 | 27 ± 4.7 | 0.007 |
| Antihypertensive medications | 634 (12%) | 162 (18%) | <0.0001 |
⁎ A total of 346 unique subjects were included in parental history group and 2,624 unique subjects were in no-parental history group.




