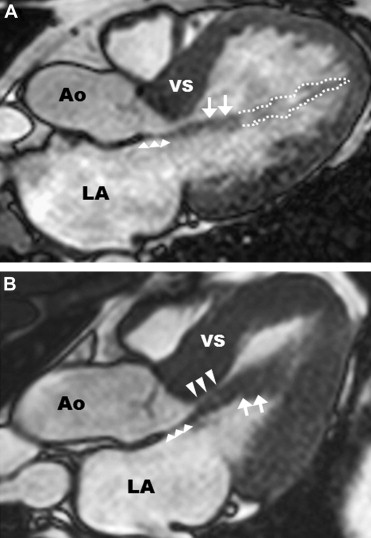
This case presents an uncommon but important mechanism of muscular left ventricular outflow obstruction in hypertrophic cardiomyopathy due to anomalous and direct papillary muscle insertion into the anterior mitral leaflet, a finding reliably identified clinically by cardiac magnetic resonance imaging. The identification of this left ventricular outflow tract morphology is important before invasive ventricular septal reduction therapy because it dictates a specific surgical strategy. These findings further support the role of cardiac magnetic resonance imaging in the early evaluation of hypertrophic cardiomyopathy patients.
Anomalous and direct papillary muscle insertion into anterior mitral leaflet is an uncommon but important mechanism of muscular left ventricular (LV) outflow tract obstruction in hypertrophic cardiomyopathy (HC), leading to progressive heart failure symptoms. Recognition of this unique structural anomaly before invasive ventricular septal reduction therapy is crucial because it dictates a specific surgical approach for septal myectomy with a deep, extended muscular resection well beyond the contact point of the mitral valve and ventricular septum. Alcohol septal ablation is unlikely to be effective in relieving LV outflow obstruction in patients with this anomaly, given that the percutaneous procedure is inflexible with the operator is confined to the fixed anatomic distribution of the first major septal perforator coronary artery. For this vignette, we studied 575 consecutive HC patients with cardiac magnetic resonance imaging (CMR) presenting to 2 major HC centers: 14 patients (2.4%) were identified with anomalous anterolateral papillary muscle insertion directly into anterior mitral leaflet ( Figure 1 ). In 13 of the 14 patients, outflow gradients of 30 to 150 mm Hg were present at rest; 4 of these patients underwent successful surgical myectomy with relief of obstruction and symptoms (including the patient presented here). Preoperative identification of this morphologic abnormality by CMR altered surgical myectomy strategy to include an extended septal resection as well as reduction in papillary muscle thickness.
Case Report
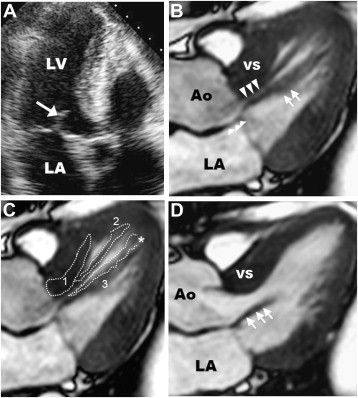
A 52-year-old man with obstructive HC and progressive heart failure was referred for invasive septal reduction therapy to relieve outflow tract obstruction. He was diagnosed with HC at age 42 (10 years earlier) after a precordial systolic ejection murmur was heard on physical examination. Over the previous 2 years, he experienced progressive exertional dyspnea (New York Heart Association functional class III) refractory to medical therapy with beta blockers and disopyramide. At the time of evaluation, a 2-dimmensional transthoracic echocardiogram demonstrated asymmetric septal hypertrophy with a maximal LV wall thickness of 17 mm (in the basal septum), mild systolic anterior motion (SAM) of the anterior mitral leaflet without septal contact ( Figure 2 ), and peak outflow gradient of 150 mm Hg. The absence of the typical mechanism of subaortic obstruction in HC (i.e., prolonged SAM-septal contact) triggered advanced imaging with CMR to further characterize the morphology of the outflow tract.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


