Fig. 5.1
Neurological pathways for pain. When the body receives a stimulus that is perceived as painful an impulse is transmitted upward from the peripheries via the spinothalamic tract to the thalamus and on to the cerebral cortex. The descending pathway is via the corticospinal tract
An example of this at its simplest would be a person who stands on a nail. The painful impulse travels up the spinothalamic tract to the brain. The brain recognizes that this is painful and wants the person to move away from the nail, so sends an instruction down the corticospinal tract so that the person moves their foot away from the nail.
Patients with a disease process may experience pain without a painful stimulus because their disease has caused damage to the ascending pathways, nerves that feed in to the ascending pathways or nociceptors found on skin, bones, joints, and other parts of the body.
Example
Miss A. has lung cancer for which she has had a lobectomy. Since the surgery she has complained of persistent chest pain near her scar. She is experiencing pain not because of a painful stimulus, but because of damage to her sensory nerves by the surgery. This sends incorrect messages to her cerebral cortex via the ascending sensory pathways and so she experiences pain.
Types of Pain
Pain can either be functional (meaning normal pain that most people experience, such as tension headache) or organic (meaning pathological and related in some way to a disease process). Organic pains can either be nociceptive or neuropathic. Nociceptive pain is due to tissue damage, whereas neuropathic pain arises from damage or disease affecting the nervous system.
Nociceptive Pain
Nociceptors are sensory receptors at the nerve ending which are sensitive to noxious or potentially noxious stimuli. They respond to such stimuli by sending a signal via the spinothalamic tract to the brain. They are found in skin, bone, muscle, connective tissues, and thoracic, abdominal, and pelvic viscera. Pain is transmitted by both Aδ and C-fibers, the latter being slower and unmyelinated. Pain transmitted through Aδ fibers is felt as a sharp and immediate pain. Pain due to activity in the C-fibers is felt as a dull, aching sensation. Visceral pain is difficult to localize, whereas other nociceptive pain can often be well localized.
Visceral pain arises when the viscera are infiltrated, compressed, distended, or stretched by an underlying disease process. This pain is often described as a deep aching pain, is poorly localized, and can be referred to unusual sites: for example, patients with lung cancer and liver metastases with associated diaphragmatic irritation may experience shoulder pain.
Neuropathic Pain
Neuropathic pain results from injury to the peripheral or central nervous system. In patients with cancer, this is commonly due to compression or infiltration of part of the nervous system by the tumor. Compression generally precedes infiltration when directly due to cancer. It may be peripheral nerves, nerve roots or the spinal cord that are involved. Damage to each of these will give pain with differing characteristics. A study of over 200 cancer patients with neuropathic pain found that 79 % had nerve compression pain, 16 % nerve injury pain, and 5 % had sympathetically maintained pain [9].
Peripheral pain is more commonly seen than central neuropathic pain. As well as being directly related to disease, neuropathic pain may result from damage secondary to surgery (e.g., peripheral nerves in the skin being cut during surgery), chemotherapy, or radiotherapy. Drugs recognized as causing peripheral neuropathy include some of those used to treat tuberculosis as well as some of the commonly used cancer treatments [10–12].
Neuropathic pain differs in nature from other pains. Typically patients describe it as a background ache with superimposed shooting or stabbing pains. Neuropathic pain can be difficult for patients to describe – they may describe it as a sandpaper-like pain, a sensation of running water but painful or like an electric shock. In general, if a patient has an unpleasant pain that they are struggling to describe or pain in a numb area, it is likely to be neuropathic pain. Central neuropathic pain is less commonly seen than peripheral pain and may be due to damage within the cerebral cortex, brainstem or spinal cord, for example spinal cord compression.
Sympathetically maintained pain is pain arising from damage to sympathetic nerves. In addition to experiencing neuropathic pain, the patient also experiences symptoms associated with activation of the sympathetic nervous system such as sweating and redness of the affected area. It can arise due to malignancy within the thorax affecting the sympathetic nerves.
Total Pain
Many pains have obvious physical causes but some do not. Pain can be perceived as being worse by the patient when, for example, they are deprived of sleep or have psychological worries. Many factors can influence pain, both in positive and negative ways. The concept of “total pain” is when physical reasons are only one factor in causing the patient’s pain. The other non-physical factors are just as valid as the physical factors, but the patient’s pain will not be resolved unless the concept of total pain is addressed.
When asked to rate the severity of their pain, patients find that scores vary throughout the day. Pain can be perceived as being worse if patients are upset or agitated, if they are bored or depressed. Patients who are lacking in sleep often perceive their pain as being more severe, as will patients who do not understand what is happening to them. Conversely, patients may feel that their pain is decreased when they are relaxed and their mind is occupied by distractions (such as visitors, reading, or activities). Patients who accept what is happening to them, have relief of other symptoms, and are sleeping well often perceive their pain as being less severe. Total pain describes how social, spiritual, psychological, and physical problems interact to result in the experience of pain (Fig.5.2). If the importance of the non-physical dimensions of the pain is not recognized, then the pain is unlikely to be adequately controlled despite analgesic medication.
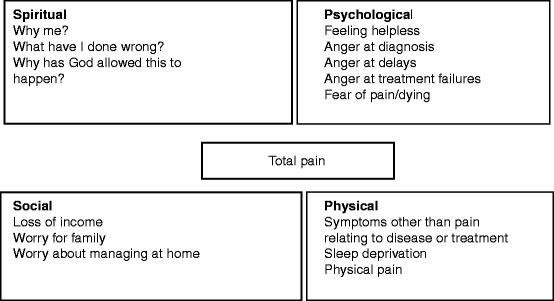

Fig. 5.2
Total pain: spiritual, psychological, social, and physical factors interact to result in the experience of pain
Regular and Breakthrough Pain
Not all pains are present all of the time. Pain that is present most of the time is termed “regular” or “background” pain and usually this requires regular analgesia. As well as this, many patients experience breakthrough or incident pain. This is pain that occurs despite regular analgesia. It is termed incident pain when it is predictable and breakthrough pain when it is not. Breakthrough pain can occur for no obvious reason but may be a source of distress to the patient. Patients may perceive this as a sign that their disease is worsening, but they should be reassured that this is not necessarily the case. Both breakthrough and incident pain require quick-acting analgesia. If the patient finds they are requiring several doses a day for breakthrough pain, this usually suggests that they are receiving inadequate regular analgesia.
Example
Mr B. has lung cancer with metastases in his pelvis. He is on morphine sulphate modified release tablets 10 mg twice daily and is pain free at rest. However, when he stands up he experiences a sudden severe pain in his pelvis radiating into his leg. As soon as he sits again, the pain resolves.
It may seem reasonable to increase his regular analgesia as he has required several rescue doses for his incident pain. However, this may well make him drowsy as he would effectively then have too much morphine in his system for the majority of the time when he is pain free. An alternative solution would need to be found whereby he just receives the extra analgesia when he needs it.
Example
Mrs C. has mesothelioma. She has had a constant painful ache over her biopsy site for several months. Since the addition of morphine sulphate modified release tablets 10 mg twice daily this had been well controlled. Recently, she has found that this pain has been coming back despite the morphine tablets. She has been taking morphine immediate release liquid with good effect, although it takes about 40 min to work. The pain recurs after a few hours. This pattern tends to happen three or four times a day. There are no obvious triggers for the flares of pain.
Mrs C’s background analgesia is no longer adequate. Her background analgesia should therefore be increased. Although her pain may have changed, she should be reassured that it does not necessarily follow that her disease has worsened.
Pain Assessment
When assessing a patient’s pain it is important to remember that pain is what the patient says it is. We all perceive pain in different ways and it is a multi-factorial symptom. Pain is often under-treated in patients with advanced disease. In the SUPPORT study, nearly 50 % of hospitalized patients reported pain and in a European study 56 % of patients reported moderate to severe pain from cancer, occurring at least monthly [13,14]. Most patients with pain have more than one pain. It is therefore important not just to ask if they have pain, but instead to ask if they have pain and how many pains they have. The site of each pain then needs to be clarified and each pain addressed in turn. There are many mnemonics that can be used in the assessment of pain. One such mnemonic is SOCRATES [15]. This leads to comprehensive questioning about the patient’s pain to ascertain how it affects the patient, the likely etiology, and the optimal treatment for that patient.
Ssite
Oonset
Ccharacter
Rradiation
Aassociated symptoms
Ttime course
Eexacerbating/relieving factors
Sseverity
Diagnosing Pain
To treat any pain successfully requires accurate diagnosis. This may involve radiological imaging or other investigations, but it is also crucial to listen carefully to the patient’s description. From this, it should be possible to determine the site of the patient’s pain, whether it radiates anywhere, and whether it is neuropathic or nociceptive.
Example
Mr D has lung cancer. He describes having pain in his left shoulder. It radiates into his arm and sometimes down his arm. He is also aware of it under his arm. The pain is often severe and feels like a constant dull ache with bouts of burning or shooting pain. It is sometimes associated with weakness.
This is a description of neuropathic pain. It is likely that the pain is coming from his brachial plexus, and since there is some associated weakness, this is probably due to compression or invasion of the brachial plexus by his cancer.
As well as considering conventional analgesia for his pain it would be appropriate to commence a short course of steroids to reduce any tumor-associated oedema that may be exacerbating the pain and also to consider whether any specific anti-cancer treatment is appropriate.
Example
Miss E is admitted to hospital with a fever and right basal lung crepitations. Her chest radiograph confirms right basal shadowing consistent with pneumonia. Her main symptoms are that cough and chest pain. Her chest pain is sharp and worse on inspiration.
The cause of the pleuritic pain is inflammation of the pleura secondary to pneumonia.
Pain Management
Principles of Pain Management
There are many different types of pain and different classes of analgesic drugs. Not all types of analgesia work for all pains. The World Health Organisation’s three step analgesic ladder has been used for many years as the method for initiating and titrating appropriate analgesia [16] (Fig.5.3). A modified version is now available specifying the need to add, rather than replace, analgesia for nociceptive pain [17].
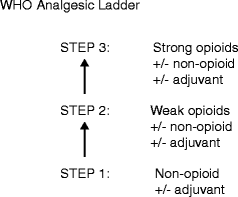

Fig. 5.3
World Health Organisation (WHO) ladder, outlining a step-wise approach to use of analgesics
Example
Mr F. has a new diagnosis of lung cancer. He is complaining of pain in his right anterior chest wall. He is awaiting an oncology multi-disciplinary team review as to what treatment he will receive. On further questioning using SOCRATES, he says that the pain started about 2 weeks ago and has gradually got worse. It is aching in nature and does not radiate. There are no associated features and he has taken the occasional paracetamol which reduces the severity from 5/10 to 3/10.
Mr F. needs regular, rather than just occasional analgesia. Taking regular paracetamol will provide more effective analgesia than he is currently receiving. Following the WHO pain ladder, if paracetamol alone does not provide adequate analgesia, it would be appropriate to add in a regular weak opioid or a non-steroidal anti-inflammatory drug.
Example
Mrs G. has ongoing pain from her mesothelioma. She is on both regular paracetamol and codeine phosphate. She finds that the severity of her pain was especially helped by the addition of ibuprofen. Her pain score reduced from 8/10 to 5/10. However, the pain still dominates her day. Her pain has not changed in nature and remains a constant aching pain.
Mrs G. is already on a weak opioid and a non-steroidal anti-inflammatory drug. Her pain seems to be visceral rather than neuropathic in nature. Following the WHO pain ladder, it would be appropriate to stop the weak opioid (codeine phosphate) and instead commence a regular strong opioid such as morphine sulphate modified release tablets.
Non-drug Treatment
Pain can be overwhelming, even when not severe. It can limit the patient’s ability to perform activities and may preoccupy their life. They may find that all they can think about is their pain. It is therefore essential to manage a patient’s pain by using non-drug methods as well as by using medication. Patients often need reassurance about their pain. They may worry that pain is an indication that their disease is worsening. This may be true, but will not be the case for all patients. To have an explanation of what is causing their pain or why it is worse is often helpful to patients. Sometimes there may be cultural beliefs associated with pain which the patient may be experiencing and they may benefit from talking these through. Careful discussion with the patient about their pain should be the first step to managing it. Having the right environment is critical to successfully managing a patient’s pain. Imagine having persistent pain and being in the middle of a busy, noisy hospital environment where you are unable to sleep properly at night. You would be tired, less able to manage the pain, and may perceive the pain as more troublesome. If, on the other hand, you were in a quiet, calm environment where you were able to adequately rest and sleep and people were available to reassure you about your pain and listen to your fears, you may be likely to perceive it as less troublesome.
Because pain can be all-consuming, the patient may find it difficult to think of anything else. A useful pain management strategy is to provide a distraction to the pain, for example a project to do, someone to talk to, questions other than about their pain. Complementary therapies can also provide a distraction from the pain, as well as relaxing the patient and may provide some analgesic benefit [18]. It is thought that acupuncture may also provide some analgesic benefit to patients, but currently there is insufficient evidence to support this [19].
TENS (transcutaneous electrical nerve stimulation) is a further possible non-drug treatment that may provide some pain relief, although, again, there is little evidence to support it [20]. Electrode pads are applied to the patient’s skin on either side of the painful area. They are connected to a machine that provides electrical stimulation to the skin. This in turn fools the brain into perceiving the pain differently. There are cautions and contraindications to using a TENS machine. In order to ensure safe use, it is essential that the TENS machine is initiated by someone who is knowledgeable and experienced in the safe use of this equipment.
Treating the underlying cause of the pain, if appropriate and possible, should always be considered. For example, if a patient has lung cancer with bone metastases and they sustain a pathological fracture, this will be painful. Treatments such as surgery or radiotherapy should be considered alongside analgesia as they may provide a more appropriate and lasting solution. Chemotherapy, radiotherapy, and surgery may all provide analgesic benefit to the patient, depending on the mechanism for their pain.
Non-opioid Analgesia
Paracetamol
Paracetamol is a centrally acting non-opioid analgesic. It blocks the enzyme cyclooxygenase (COX) and so inhibits the production of prostaglandins which are implicated in pain mechanisms [21]. There are two main iso-enzymes of COX which are expressed to varying levels by different tissues. COX-1 is produced constitutively, for example, in gastric mucosa, whereas COX-2 is undetectable in normal tissues but is highly inducible, for example, at sites of inflammation and cancer.
Although the effects of paracetamol are similar to COX inhibitors, it only has anti-pyretic properties and does not share the anti-inflammatory actions that they also possess [22]. Taking paracetamol regularly should be more effective than just taking it as required because it enables ongoing suppression of prostaglandin synthesis and thereby ongoing reduction in pain.
There is contradictory evidence about whether or not paracetamol has a synergistic effect when used with strong opioids: one study showed no benefit when paracetamol versus placebo was added to strong opioids [23]; another demonstrated a clinically important benefit in about one-third of patients [24]. It would therefore seem sensible to either continue or start paracetamol as a trial in patients receiving strong opioids. If their pain does not improve within a few days of adding in paracetamol, it should be stopped. Paracetamol comes in 500 mg tablets and the regular dose is 1 g four times a day. Continuing regular paracetamol tablets therefore requires that the patient takes eight additional tablets per day. This is a significant increase on an often already hefty pill-burden.
Adverse Effects: Paracetamol rarely causes adverse effects. The main concern is that of hepatotoxicity in overdose.
Non-steroidal Anti-inflammatory Drugs (NSAIDs)
As with paracetamol, NSAIDs work by inhibiting the enzyme cyclooxygenase (COX). However, in addition to anti-pyretic properties, they also have anti-inflammatory effects [25]. The original NSAIDs inhibited both COX-1 and COX-2, whereas some of the newer ones are more selective for COX-2 [26]. The COX-1 enzyme is produced constitutively (e.g., in gastric mucosa), whereas COX-2 is highly inducible (e.g., at sites of inflammation and cancer).
Examples of NSAIDs and Their Preferences and Selectivity
Preferential for COX-1, e.g., ketorolac
Preferential for COX-2, e.g., diclofenac
Selective for COX-2, e.g., the coxibs – celecoxib, rofecoxib
Non-selective, e.g., aspirin, ibuprofen
Adverse Effects: Although NSAIDs are very effective painkillers, they must be used with caution as they have several potentially significant adverse effects.
Gastro–duodenal Irritation: NSAIDs are well recognized to cause gastro-duodenal irritation. This is predominantly due to COX-1 effects, and so selective COX-2 inhibitors were developed which had a lower incidence of this adverse effect [27]. Unfortunately, one of the coxibs (rofecoxib) led to a significantly increased rate of prothrombotic events. Other studies confirmed that this may be true of all coxibs and not just limited to rofecoxib [28,29]. To reduce the likelihood of gastro-duodenal adverse effects, an NSAID should be chosen that has a lower risk of such adverse effects and be used in combination with a gastro-protective agent [30].
Bronchospasm: Caution is needed in using NSAIDs in patients with asthma as they can provoke bronchospasm [31].
Renal toxicity: Acute renal failure secondary to NSAIDs occurs in less than 1 % of patients given an NSAID per year. It is more likely if patients are dehydrated [32]. This risk is highest within the first 30 days of initiating treatment and reduces thereafter. It occurs with similar association across the different classes of NSAIDs [33].
There are many examples of NSAIDs with varying profiles and it can be difficult to know which to select for a patient. Thought must be given to the individual patient, including their likelihood of adverse effects, and then an appropriate drug chosen. Consideration must also be given to co-prescribing a proton-pump inhibitor as gastro-protection.
Opioid Analgesia
The WHO analgesic ladder divides opioids into weak and strong. This suggests that the two classes have distinct properties. The opioids share similar adverse effects and high-dose codeine is equivalent to low-dose morphine for analgesia. It may be more accurate to classify opioids along a gradient rather than separating them in this way.
Weak Opioids
Weak opioids are said to have a “ceiling effect” for analgesia, meaning that there is a dose beyond which the patient gets more adverse effects without any additional analgesic benefit. It is probably more accurate to say that as doses increase so does the likelihood of adverse effects (especially nausea and vomiting) and this outweighs any additional analgesic benefit.
When prescribing a weak opioid, the WHO analgesic ladder should be followed: a weak opioid should be added to other analgesia and not replace it (assuming it has provided some analgesic benefit). If a weak opioid is being used regularly but the patient is still experiencing some pain, then the weak opioid should be stopped and the patient moved to step 3 of the analgesic ladder and given a strong opioid instead of the weak opioid. There is no real difference in analgesic properties between the weak opioids, so if one is not providing sufficient analgesia there is no benefit in switching to an alternative weak opioid. Similarly, there is no benefit in giving a weak opioid to patients already receiving a strong opioid. Commonly used weak opioids include codeine phosphate, dihydrocodeine, and tramadol. Approximate dose conversions are shown below [34]. Tramadol is a synthetic analogue of codeine and therefore considered as a weak opioid. It also inhibits serotonin and noradrenaline (norepinephrine) re-uptake [35].
Approximate Oral Opioid Potency Ratios
Opioid | Potency ration with morphine | Dose equivalence to 10 mg morphine (mg) |
|---|---|---|
Codeine | 1/10 | 100 |
Dihydrocodeine | 1/10 | 100 |
Tramadol | 1/10 | 100 |
Conversions are approximations because of individual variation in pharmacokinetics. They should be used as a guide and the patient should always be closely assessed after performing a switch.
Strong Opioids
If a patient has tried weak opioids and their pain persists, or if they have severe pain, they should be started on a strong opioid. One common concern of prescribers, especially for patients with respiratory conditions, is that strong opioids may cause clinically significant respiratory depression. This is not the case. Pain is a respiratory stimulus, even when being treated by morphine, and it therefore antagonizes opioid-induced respiratory depression [36]. Opioids must, however, be used in an appropriate fashion – the patient should be started on a low dose (or equi-analgesic dose if already on a different opioid) and the dose of strong opioid titrated upward according to response. The body has several different subtypes of opioid receptor and different opioids work preferentially at the different receptors. However, this does not tend to guide prescribing. It should be more influenced by the patient’s pre-existing medical conditions, adverse effect profile, and ease of administration for the patient.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


