Chapter 10 Paediatrics
INTRODUCTION
The principal reason for hospital admissions in children aged 0–4 years is respiratory illness and the management of children with acute or chronic respiratory disorders has become a specialized area of respiratory physiotherapy. The inexperienced physiotherapist working with children will require the support and mentorship of an experienced paediatric physiotherapist in order to develop the necessary skills.
DEVELOPMENT OF THE LUNGS
The development of the lung can be divided into four stages (Inselman & Mellins 1981):
Pseudoglandular period (weeks 6–16).
During this period the airways grow by dichotomous branching so that by week 16 all generations of the airway from trachea to terminal bronchioles (i.e. the preacinus) are formed. During this period the pulmonary circulation also develops, cartilage and lymphatic formation occur and cilia appear (week 10 onwards) (Langman 1977).
Canalicular period (weeks 17–24).
The respiratory bronchioles, alveolar ducts and alveoli (i.e. the acinus) start to develop during this time, simultaneously with the lung capillaries, thus preparing the lungs for their future role in gas exchange (Hislop & Reid 1974). The air–blood barrier first appears at week 19 and towards the end of this period surfactant synthesis begins.
Terminal sac period (week 24–term).
Development of the pulmonary circulation continues and the respiratory bronchioles subdivide to form air spaces. Two different cell types (types I and II pneumocytes) line the air spaces. Type I pneumocytes flatten and elongate to cover the majority of the surface area of the saccular air spaces. Type II cells only occupy approximately 2% of the surface and are responsible for surfactant synthesis and storage (Greenough 1996). Surfactant is a phospholipid, which stabilizes surface tension in the alveolus and prevents alveolar collapse on expiration. Small quantities of surfactant are present at weeks 23–24 of gestation and the amount present gradually increases until a surge at about week 30. Birth itself and the onset of respiration stimulate surfactant production.
Towards the end of the terminal sac period, the air spaces have developed into primitive multilocular alveoli. After birth, alveoli increase in size and number. The average number of alveoli in the newborn is 150 million. By the age of 3–4 years, the adult number of 300–400 million alveoli has been reached, but alveolar growth continues for the first 7 years (Hislop et al 1986). More recent estimations of mean alveolar number in adulthood have been 480 million (range 274–790 million), with alveolar number closely related to lung volume (Ochs 2004).
RESPIRATORY SYSTEM: ANATOMICAL AND PHYSIOLOGICAL DIFFERENCES BETWEEN CHILDREN AND ADULTS
Anatomical differences
Rib cage and chest shape
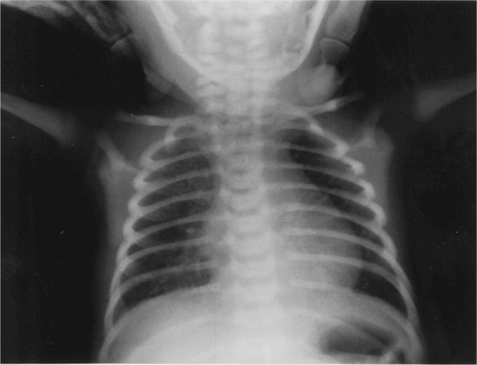
The cross-sectional shape of the infant thorax is cylindrical and not elliptical as in adolescents or adults. The ribs of the newborn infant are relatively soft and cartilaginous compared with the more rigid chest wall of older children and adults. They are also placed horizontally in relation to the sternum and vertebral column compared with the more oblique rib angle of adults (Fig. 10.1). The bucket handle rib movement seen in older children and adults is therefore not possible. As the infant grows, and begins to develop an upright posture, the ribs develop a more oblique angle and the transverse diameter of the rib cage increases. The adult chest shape is achieved by 3 years of age (Openshaw et al 1984).
The intercostal muscles are poorly developed in infancy and contraction of the intercostal muscles is inefficient at improving thoracic volumes either by increasing the anteroposterior or transverse diameters of the chest. Increased ventilatory requirements have to be met by increasing the respiratory rate rather than depth (Konno & Mead 1967).
Diaphragm
Maximal diaphragmatic activity during severe respiratory distress or respiratory obstruction leads to an inward movement of the lower rib cage instead of a downward movement of the diaphragm, as well as intercostal and sternal recession (Muller & Bryan 1979). Despite these disadvantages, the diaphragm is the main muscle of inspiration in the infant, since the intercostals are poorly developed. Ventilation in the infant is also more affected by impaired diaphragmatic function, for example by abdominal distension, hepatomegaly or phrenic nerve damage.
Preferential nasal breathing
The shape and orientation of head and neck in babies (large head, prominent occiput, short neck, large tongue, smaller retracted lower jaw, high larynx) mean that the airway is prone to obstruction in young infants. Young infants up to about 6 months of age are preferential nasal breathers and studies suggest that up to half of all neonates are unable to breathe through their mouths, except when crying, for the first few weeks of life (King & Booker 2004) The small nasal passages account for between 30% and 50% of the total airway resistance in neonates. The narrowest portion of the nasal airway has a cross-sectional area of about 20 mm2. Therefore, even a small amount of swelling or obstruction of the nasal passages of infants compromises breathing considerably and causes a disproportionate and detrimental effect on the work of breathing. Some young infants with upper respiratory tract infections and partial obstruction of their nasal passages can develop respiratory distress.
Airway diameter
At birth there is no further increase in the number of airways formed but there is growth and development in their size. In the first few years of life there is a significant increase in the diameter of the larger, more proximal airways (Hislop & Reid 1974). The smaller, more distal airways do not increase in diameter until nearer 5 years of age. This higher peripheral airways resistance is exacerbated by respiratory infections, which cause inflammation of the airways, for example in bronchiolitis, or in the presence of secretions.
Bronchial walls
The bronchial walls are supported by cartilage, which begins to develop from 12 weeks’ gestation and continues throughout childhood. The cartilaginous support of an infant’s airways is much less than that of an adult, and predisposes the airways to collapse. The bronchial walls contain proportionally more cartilage, connective tissue and mucous glands than do those of adults, but less smooth muscle; this makes the lung tissue less compliant. The lack of bronchial smooth muscle, particularly in the smaller bronchioles, may be one reason for the lack of response to bronchodilators under the age of 12 months. The β-receptors in infants are also immature, which further reduces any response to β-adrenergic bronchodilator therapy (Reid 1984). The high proportion of mucous glands in the major bronchi of infants makes the airways more susceptible to mucus obstruction.
Alveoli and surfactant
The respiratory system is not fully developed at birth, even in the term neonate, and postnatal maturation continues for a significant time. Although by 20–27 weeks’ gestation lung acinar have formed, several types of epithelial cells can be differentiated, and the air–blood barrier is thin enough to support gas exchange; true alveoli develop only after about 36 weeks’ gestation. A term newborn has an average of 150 million alveoli. The remainder of the eventual average of 400 million alveoli develop after birth, the vast majority within the first 2 years of life. Both the number and size of alveoli continue to increase postnatally until the chest wall stops growing. By 4 years of age, the adult number of 300 million may exist, although growth can continue until 7 years of age. The smaller alveolar size of an infant makes the infant more susceptible to alveolar collapse, and the smaller number of alveoli reduces the area available for gaseous exchange (Reid 1984).
Pulmonary surfactant is a mixture of phospholipids (90%) and apoproteins (10%), which act to reduce surface tension at the air–liquid interface in the alveolus, thereby preventing collapse of lung parenchyma at the end of expiration. Type II alveolar cells synthesize and secrete surfactant from 23 to 24 weeks’ gestation. In preterm newborns, a deficiency of surfactant is a major factor in the development of neonatal respiratory distress syndrome (RDS). Male gender is a risk factor for neonatal RDS, bronchopulmonary dysplasia (BPD) and mortality. Boys with neonatal RDS seem to have more health problems than girls during the neonatal period.
Collateral ventilation
Collateral ventilation is the means by which a distal lung unit can be ventilated, despite blockage of its main airway. Collateral ventilatory pathways are achieved by a network of interconnecting pathways linking different structures. Respiratory bronchioles are linked by channels of Martin. Canals of Lambert connect respiratory and terminal bronchioles with alveoli and their ducts; and adjacent alveoli are joined by openings in the alveolar wall, called pores of Kohn (Menkes & Traystman 1977). However, none of these pathways exists at birth. The pores of Kohn develop between years 1 and 2, and the canals of Lambert do not appear until about 6 years of age. The collateral ventilatory channels between alveoli, respiratory bronchioles and terminal bronchioles are poorly developed until 2 and 3 years of age, predisposing towards alveolar collapse.
Height and exposure to air pollution
Because children breathe more rapidly compared with adults and because they spend more time outdoors being physically active, they tend to be more exposed to outdoor air pollution and allergens than do adults and have greater deposition of particulate matter. Their reduced height means they are also more exposed to vehicle exhausts and heavier pollutants that concentrate at lower levels in the air. There is substantial evidence linking air pollution with respiratory health problems and children are more vulnerable (Brauer et al 2007, Pénard-Morand et al 2005).
Physiological differences
Closing volume
The closing volume is the lung volume at which closure of the small airways occurs. This volume plus the residual volume (the volume of gas left in the lungs following maximum expiration) is known as the closing capacity (CC). In the adult, CC is less than FRC, i.e. the volume of gas left in the lungs following tidal expiration, whereas in the infant it is greater than FRC. The higher closing volumes apparent in infants are due to greater chest wall compliance and reduced elastic recoil of the lungs than in the adult. Therefore, airway closure may occur before the end of expiration, e.g. during expiratory chest vibrations, putting the infant at a much greater risk of developing widespread atelectasis, especially in the presence of lung disease, where lung volume is further reduced. In the event of respiratory distress, the infant grunts on expiration, adducting the vocal cords in an attempt to reduce the amount of gas expired, thus maintaining a higher FRC and minimizing alveolar collapse (Pang & Mellins 1975). Re-inflation of alveoli, once collapsed, is more difficult in the infant, who has to work considerably harder to overcome the effects of the compliant chest wall.
Ventilation and perfusion
In the adult, both ventilation and perfusion are preferentially distributed to the dependent lung. The best gas exchange and ventilation/perfusion match will therefore be in the dependent region of the lung (Zack et al 1974). In the infant, however, ventilation is preferentially distributed to the uppermost lung (Davies et al 1985), whereas the perfusion remains best in the dependent regions. This leads to greater gas exchange in the uppermost lung (Heaf et al 1983) but an imbalance between ventilation and perfusion (Bhuyan et al 1989). In acutely ill children with unilateral lung disease, oxygenation may be optimized by placing the ‘good’ lung uppermost. However, this is contrary to the goal of improving ventilation to the diseased lung and facilitating secretion clearance, in which positioning and postural drainage would require the diseased lung to be uppermost. The therapist would have to balance their decision based on the stability, tolerance and current therapeutic priorities.
The difference in ventilation distribution between infants and adults is most likely due to the more compliant rib cage of the infant, which compresses the dependent areas of lung. In addition, while in the adult the weight of the abdominal contents provides a preferential load on the dependent diaphragm and therefore improves its contractility, in the infant this does not happen. The effect on both hemidiaphragms is similar, due to the abdomen being so much smaller and narrower (Davies et al 1985). It has been shown in adults that, when the diaphragm is inactivated, e.g. when ventilated under anaesthetic, the ventilation distribution changes to that of an infant (Rehder et al 1972). It is not yet known exactly when the ventilation distribution in the infant changes to that of an adult, but it may be as late as 10 years of age.
Oxygen consumption, cardiac output and response to hypoxia
Although anatomical closure of the foramen ovale can occur as early as 3 months of age, the channel remains ‘probe patent’ in 50% of children up to 5 years of age, and persists in about 30% of adults. Similarly, anatomical closure of the ductus arteriosus usually occurs between 4 and 8 weeks of age. Any stimulus, such as hypoxia or acidosis, that causes an increase in pulmonary vascular resistance during the neonatal period may allow these two potential channels to reopen, resulting in right-to-left shunting and increasing hypoxia (King & Booker 2004).
Muscle fatigue
The respiratory muscles of infants tire more quickly than those of adults due to a much smaller proportion of fatigue-resistant muscle fibre (Keens & Ianuzzo 1979). There are two main muscle fibre types, type I and type II. Type I muscle fibres are slow twitch, high oxidative and slow to fatigue. Type II fibres are fast twitch, slow oxidative and tire quickly. Of the muscle fibres in the adult diaphragm, 55% are type I compared with only 30% in the infant. Premature infants tire even more easily as, at 24 weeks’ gestation, only 10% of their muscle fibres are fatigue resistant (Muller & Bryan 1979). Excessive muscle fatigue results in apnoea. By 12 months of age the number of type I fibres equals that of an adult.
Breathing pattern and rapid eye movement sleep
During rapid eye movement (REM) sleep there is a reduction in postural tone and tonic inhibition of the infant’s intercostal muscles such that the rib cage is even less well equipped to counteract the contraction of the diaphragm during inspiration (Muller & Bryan 1979). This reduces the efficiency of respiration, causes a drop in functional residual capacity and increases the work of breathing, predisposing the infant to apnoeic episodes (Muller & Bryan 1979). The premature infant is most at risk, spending up to 20 hours a day asleep, 80% of which may be in active REM sleep compared with 20% in adult sleep.
Response to cold
Paediatric patients have an increased surface area per kilogram and lose heat to the environment more readily than adults. This is compounded by cold intravenous fluids, dry anaesthetic gases and exposure. Non-shivering thermogenesis in brown adipose tissue is the major mechanism of heat production during the first few months of life. Brown fat is specialized tissue located in the posterior of the neck, along the interscapular and vertebral areas, and surrounding the kidneys and adrenal glands. Metabolic heat production can increase up to two and a half times during cold stress. Shivering is a less economical form of heat production but does occur in severely hypothermic neonates. Hypothermia is a serious problem that can result in increased oxygen consumption, cardiac irritability and respiratory depression (King & Booker 2004).
RESPIRATORY ASSESSMENT OF THE INFANT AND CHILD
Careful assessment is essential to identify problems requiring physiotherapy intervention. Many aspects of assessment will be the same as in adults (Chapter 1), but specific differences are listed below.
Discussion with the relevant carers
When assessing the hospitalized child, information should be obtained about:
 whether the child is fed via the oral, nasogastric or intravenous route and the timing of the last feed
whether the child is fed via the oral, nasogastric or intravenous route and the timing of the last feedObservation charts and investigations
Results of investigations and other relevant observations should be referred to as appropriate.
Examination
Examination of the older child is similar to that of the adult (Chapter 1). The following specific factors should be considered in younger children.
Clinical signs
Clinical signs of respiratory distress are listed in Box 10.1.
Recession occurs when high negative intrathoracic pressure during inspiration pulls the soft, compliant chest wall inward. It may be sternal, subcostal or intercostal. Mild recession may be normal in preterm infants but in older infants is a sign of increased respiratory effort.
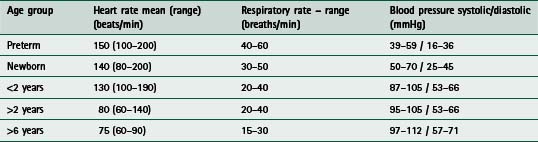
Tachypnoea (respiratory rate greater than 60 breaths/min) may indicate respiratory distress in infants. Normal values are listed in Table 10.1.
PHYSIOTHERAPY TECHNIQUES IN INFANTS AND CHILDREN
Most physiotherapy techniques used in adults can be applied in children and the same contraindications apply (Chapters 5 & 6). Treatment should never be performed routinely as it may have potentially detrimental effects (Horiuchi et al 1997, Krause & Hoehn 2000, Stiller 2000). Ideally treatment should occur before feeds or adequate time allowed following a feed to avoid problems associated with vomiting and aspiration.
Chest percussion
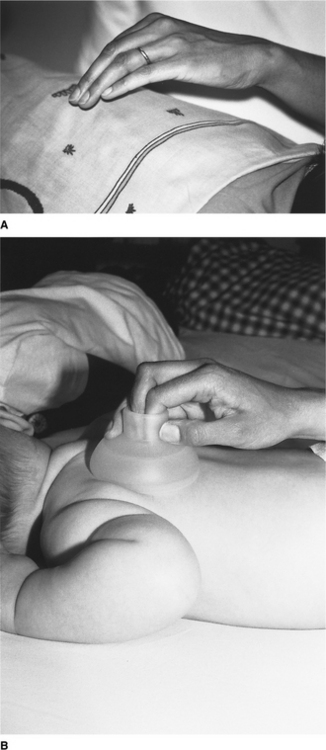
Chest percussion (sometimes referred to as chest clapping) using the hand, fingers or a facemask is generally well tolerated and widely used in children. Percussion with one hand is used in small children and babies (Fig. 10.2A). In neonates and preterm infants ‘tenting’ (using the first three or four fingers of one hand with slight elevation of the middle finger) or the use of a soft plastic cup-shaped object such as a facemask may be more appropriate (Fig. 10.2B) (Tudehope & Bagley 1980).
Vibrations and shaking
Chest wall vibrations involve the application of a rapid extrathoracic compressive force at the beginning of expiration, followed by oscillatory compressions until expiration is complete. The compressions and oscillations applied during chest wall vibrations are believed to aid secretion clearance via a number of physiological mechanisms, including increasing peak expiratory flow to move secretions towards the large airways for removal by suction or cough (Kim et al 1987, King 1998, McCarren et al 2006, Ntoumenopoulos 2005, van der Schans et al 1999, Wanner 1984).
Chest wall vibrations remain objectively undefined and may vary considerably between practitioners and units. The terms chest vibrations, compressions, shaking and expiratory flow increase techniques have been used variously in the literature (Almeida et al 2005, Sutton et al 1985, Wong et al 2003).
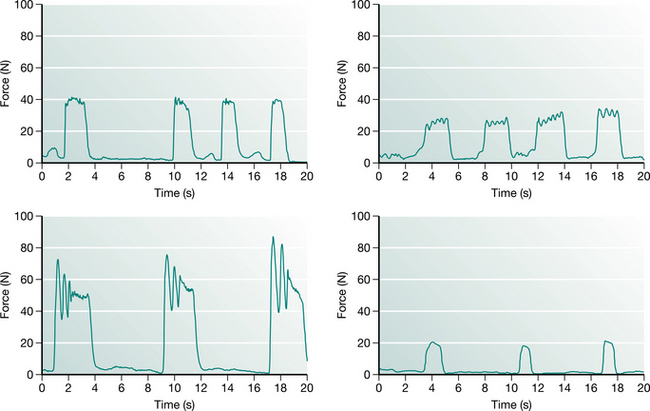
Chest wall vibrations appear to be used more frequently in ventilated children than percussion, probably because the glottis is held open by the endotracheal tube, facilitating rapid expiratory flow during vibrations that improve mucus clearance. There is a strong linear relationship between the maximum force applied during chest wall vibrations and the age of the child, most likely reflecting modification of techniques to accommodate changes in chest wall compliance (Gregson et al 2007a). Maximum force applied during physiotherapy can vary substantially between physiotherapists. Similarly there is marked variability in the pattern of force–time profiles between physiotherapists with respect to the duration of vibration, and amplitude, number and frequency of oscillations. Figure 10.3 illustrates the style of force profiles delivered to four infants, all aged between 5 and 14 months by four different physiotherapists. However, there is remarkable consistency within and between each physiotherapist’s treatment sessions (Gregson et al 2007b). The clinical consequences for such variation in treatment profiles remain unclear.
Precautions for chest percussion and vibratory techniques
Chest percussion has been reported to cause an increase in bronchospasm in adults with chronic lung disease (Campbell et al 1975, Wollmer et al 1985). Premedication with bronchodilator therapy may reduce this effect but in severe cases percussion should be avoided.
Postural drainage (gravity-assisted positioning)
The use of gravity-assisted positioning, including a head-down tip, has traditionally been a component of airway clearance in babies and children. However, the use of the head-down tipped position has been the focus of considerable debate in recent years. Very few studies have examined specifically the efficacy of gravity-assisted positioning in infants and children. An Australian study of 20 babies with cystic fibrosis reported an increase in gastro-oesophageal reflux in those receiving postural drainage (PD) using a head-down tipped position compared with modified PD without a head-down tilt (Button et al 1997). Another study also undertaken in babies with cystic fibrosis (CF) (Phillips 1996) reported no adverse effect of the head-down tipped position on gastroesophageal reflux. This discrepancy could be attributed to the differences between the two study populations. Despite the inconsistency between these two studies, the concerns raised have led to a significant change in practice in many CF centres. This has to some extent been extrapolated to other paediatric respiratory disorders with the result that the head-down tipped position is now used much less in paediatric practice. A head-down tip should never be used in children with raised intracranial pressure or in preterm infants because of the risk of periventricular haemorrhage. Abdominal distension places the diaphragm at a mechanical disadvantage and a head-down tilt is likely to exacerbate this further.
Positioning
Positioning may be used to optimize respiratory function. The supine position has been shown to be the least beneficial, while prone positioning has been shown to improve respiratory function (Chapter 4), decrease gastro-oesophageal reflux (Blumenthal & Lealman 1982) and reduce energy expenditure (Brackbill et al 1973). It is often used in closely monitored infants with respiratory problems in a hospital setting, but parents should be advised against using this position when babies are sleeping unattended because of its association with sudden infant death (Southall & Samuels 1992).
Patterns of regional ventilation in infants differ significantly from adults (Davies et al 1985), with ventilation in infants and small children being preferentially distributed to the uppermost regions of the lungs. In acutely ill children with unilateral lung disease, care should be taken if positioning the child with the affected lung uppermost as this may cause rapid deterioration of respiratory status. Spontaneously breathing newborn infants are better oxygenated when tilted slightly head up (Thoresen et al 1988) and show a drop in PaO2 if placed flat or tilted head down.
It is suggested that the redistribution of ventilation, which occurs with a change in body position, results in optimized ventilation to specific lung regions and localized improvement in airway patency. This may result in enhanced secretion clearance from these regions, which are not necessarily those positioned in such a way to allow gravitational drainage (Lannefors & Wollmer 1992).
Manual ventilation
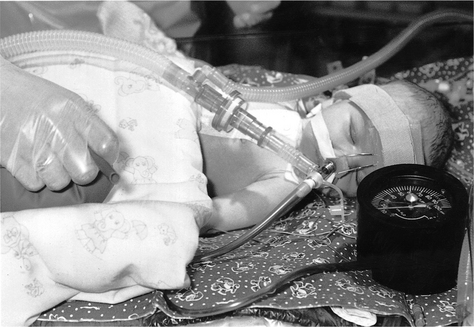
Manual lung inflation involves disconnection of the patient from mechanical ventilation to provide temporary manual ventilation. The same contraindications apply for children and adults (Chapters 5 & 8). However, special consideration should be applied in preterm infants whose lung tissue is easily damaged by high inflation pressures and in children with hyperinflated lungs (e.g. asthma and bronchiolitis) in whom there is a greater risk of pneumothorax. For infants, 500 ml bags should be used and 1 litre bags for older children. They may be valved or open-ended, so that expulsion of excess pressure is controlled by the operator’s fingers. A manometer should be placed in the circuit whenever possible to monitor the inflation pressures (Fig. 10.4). As a general guideline, manual ventilation pressures during physiotherapy should not exceed 10 cmH2O above the ventilator pressure. In order to prevent airway collapse, some positive end-expiratory pressure (PEEP) should be maintained in the bag. Self-inflating bags are used in some units. The flow rate of gas is adjusted according to the size of the child: 4 l/min for infants increasing to 8 l/min for children.
In paediatric patients manual ventilation is used to achieve the following:
Hyperinflation – a long inspiration with an inspiratory pause followed by rapid release of the bag. The aim of this technique is to recruit lung units by improving collateral ventilation and increasing lung volume. However in acute respiratory distress, the proportion of recruitable lung may be extremely variable (Gattinoni et al 2006). Following hyperinflation, a high expiratory flow may assist in mobilizing secretions towards central airways. Some studies support the use of hyperinflation for improving respiratory mechanics (Choi & Jones 2005, Marcus et al 2002). However there remains some controversy over the safety and effectiveness of manual lung hyperinflation as the volumes, pressures and FiO2 are not always controlled and there are inherent dangers of barotrauma (Berney & Denehy 2002, Gattinoni et al 1993, Savian et al 2006). In children with compromised cardiac output, the long inspiratory phase with pause may be contraindicated.
Hyperoxygenation – may be used before suction in order to reduce suction-induced hypoxia or pulmonary hypertension. A review of the efficacy of ventilator versus manual hyperinflation in delivering hyperoxygenation or hyperinflation breaths before, during and/or after endotracheal suctioning found that hyperoxygenation or hyperinflation breaths at 100% oxygen delivered via the ventilator were either superior or equivalent to manually delivered breaths in preventing suction-induced hypoxaemia. However, delivery of manual hyperinflation breaths resulted in increased airway pressure and increased haemodynamic consequences (Stone 1990, Stone & Turner 1989). In the presence of pulmonary hypertension, it is generally not advisable to use an FiO2 of 1.0 during manual hyperinflation as this may further increase blood flow to the lungs.
Independently performed airway clearance techniques
Over the past two decades, several modalities of airway clearance have been developed. The aim of all of these techniques is to effectively enhance clearance of bronchial secretions and at the same time to facilitate independence with treatment. The majority of techniques were developed for chronic lung disease, in particular cystic fibrosis, but their use has become widespread in both acute and chronic disorders and they are commonly used in paediatric practice (Fig. 10.5). The various techniques are described in detail in Chapter 5 and include:
Airway clearance for children with neurological and neuromuscular impairment
Impaired cough, as a consequence of weakness from neuromuscular disease such as Duchenne muscular dystrophy and spinal muscular atrophy or neurological impairment, can cause serious respiratory complications including atelectasis, pneumonia, airway obstruction and acidosis (Miske et al 2004). Chronic respiratory insufficiency and respiratory failure will ultimately result from chronic weakness of respiratory muscles, shallow breathing and ineffective cough. For these children, independently performed airway clearance techniques are not usually feasible, but options such as the ‘cough assist’ (mechanical insufflation/exsufflation device) and other non-invasive forms of positive pressure ventilation are safe and well tolerated in this client group, with growing evidence to support their efficacy (Chatwin et al 2003, Panitch 2006, Vianello et al 2005). They are discussed more comprehensively in Chapters 5 &11. Not all patients with neuromuscular disease are good candidates for the use of non-invasive respiratory aids. Potential contraindications include an inability to manage oropharyngeal secretions, mental status changes or cognitive impairment, and cardiovascular instability. For some patients, including those with the most severe spinal muscular atrophy, sole reliance on non-invasive methods of assisted cough and ventilation is inadequate, and they may require repeated episodes of intubation and mechanical ventilation in the intensive care unit to prolong survival (Birnkrant 2002).
Breathing exercises
It is possible to encourage children to deep breathe from about 2 years of age by using games such as bubbles, paper windmills or incentive spirometers, although the efficacy of these treatments is unproven. Laughing is a very effective means of lung expansion in infants. As children get older, they are able to play a more active role in their treatment and appropriate airway clearance techniques can be introduced (Chapter 5).
Airway suction
Airway suction is discussed in Chapters 5 and 8. Suction techniques may be either naso- or oropharyngeal or endotracheal, depending on whether there is an artificial airway in situ. Adverse effects have frequently been reported and include hypoxaemia, mechanical trauma, apnoea, bronchospasm, pneumothorax, atelec-tasis, cardiac arrhythmias and even death on rare occasions (Clark et al 1990, Clarke et al 1999, Czarnik et al 1991, Kerem et al 1990, Shah et al 1992, Singer et al 1994, Stone & Turner 1989, Wood 1998). Practice varies widely among centres and where available local guidelines should be taken in to consideration (Sole et al 2003).
Complications associated with suction may be reduced by:
 Preoxygenation before suction using ventilator or manually delivered breaths with a higher FiO2 (Chulay & Graeber 1988, Goodnough 1985). Preoxygenation with ventilator breaths has been recommended in preference to disconnection and manual hyperinflation because of the reduced risk of barotrauma, loss of PEEP and FiO2 (Glass et al 1993, McCabe & Smeltzer 1993, Stone et al 1991). Particular care should be taken in preterm infants to avoid hyperoxia, as this is associated with retinopathy of prematurity (Roberton 1996).
Preoxygenation before suction using ventilator or manually delivered breaths with a higher FiO2 (Chulay & Graeber 1988, Goodnough 1985). Preoxygenation with ventilator breaths has been recommended in preference to disconnection and manual hyperinflation because of the reduced risk of barotrauma, loss of PEEP and FiO2 (Glass et al 1993, McCabe & Smeltzer 1993, Stone et al 1991). Particular care should be taken in preterm infants to avoid hyperoxia, as this is associated with retinopathy of prematurity (Roberton 1996). Suctioning via a port adapter or closed suction systems in patients who require maintenance of PEEP and/or positive pressure ventilation during suction (Harshbarger et al 1992).
Suctioning via a port adapter or closed suction systems in patients who require maintenance of PEEP and/or positive pressure ventilation during suction (Harshbarger et al 1992). Avoiding cross-infection, particularly in vulnerable infants, by meticulous hand washing and adherence to local infection control policies.
Avoiding cross-infection, particularly in vulnerable infants, by meticulous hand washing and adherence to local infection control policies. Keeping suction pressures as low as possible, without compromising the efficacy of secretion clearance. High vacuum pressures have been associated with mechanical trauma of the tracheal mucous membranes (Kleiber et al 1988).
Keeping suction pressures as low as possible, without compromising the efficacy of secretion clearance. High vacuum pressures have been associated with mechanical trauma of the tracheal mucous membranes (Kleiber et al 1988). Selecting a suction catheter with an external diameter which does not exceed 50% of the internal diameter of the airway (Imle & Klemic 1989). Most commonly used catheters are 6 and 8 French gauge (FG). Size 5 FG and below are usually ineffective in removing thick secretions. Size 10 FG and above should be reserved for use with older children.
Selecting a suction catheter with an external diameter which does not exceed 50% of the internal diameter of the airway (Imle & Klemic 1989). Most commonly used catheters are 6 and 8 French gauge (FG). Size 5 FG and below are usually ineffective in removing thick secretions. Size 10 FG and above should be reserved for use with older children. Using graduated catheters with centimetre markings to gauge how far the catheter has been passed. Pneumothorax due to direct perforation of a segmental bronchus by a suction catheter has been reported in intubated preterm infants (Vaughan et al 1978).
Using graduated catheters with centimetre markings to gauge how far the catheter has been passed. Pneumothorax due to direct perforation of a segmental bronchus by a suction catheter has been reported in intubated preterm infants (Vaughan et al 1978). Positioning in side lying and restraining the non-intubated child who requires nasopharyngeal suction, to avoid potential aspiration of gastric contents (Fig. 10.6). Constant reassurance should be given throughout the procedure. Supplemental oxygenation and resuscitation equipment should be available. Naso- pharyngeal suction of neonates may cause reflex bradycardia and apnoea.
Positioning in side lying and restraining the non-intubated child who requires nasopharyngeal suction, to avoid potential aspiration of gastric contents (Fig. 10.6). Constant reassurance should be given throughout the procedure. Supplemental oxygenation and resuscitation equipment should be available. Naso- pharyngeal suction of neonates may cause reflex bradycardia and apnoea. Avoiding nasopharyngeal suction if the child has stridor or has recently been extubated, as this may precipitate laryngospasm.
Avoiding nasopharyngeal suction if the child has stridor or has recently been extubated, as this may precipitate laryngospasm.Saline instillation
Saline instillation into the tracheal tube of ventilated patients aims to loosen thick or sticky secretions to facilitate easy removal with suction (Schreuder & Jones 2004). Evidence for the practice is variable and therefore saline should be used only where there is a clear indication. Some suggest that saline instillation at best is not effective and at worst is harmful (Blackwood 1999, Hagler & Traver 1994, Kinloch 1999, McKelvie 1998, Ridling et al 2003), while others suggest it is well tolerated even in infants and may be helpful in removing secretions adherent to the chest wall (Shorten et al 1991). Other mucolytics (N-acetylcysteine) in aliquots of 0.5–5 ml may be used to enhance secretion clearance. Larger quantities of irrigants are sometimes used as part of bronchoalveolar lavage procedures.
RESPIRATORY DISEASE IN CHILDHOOD
Respiratory disease in childhood is very common and is one of the major causes of morbidity and mortality in children worldwide. Outside of the developing countries, most illnesses are mild; only a small proportion are more serious, involving the lower respiratory tract. The overall mortality rate per 100 000 children aged between 1–16 years due to respiratory illness in England and Wales has declined from 8.6 in 1968 to 1.3 in 2000. Asthma, pneumonia and cystic fibrosis (CF) together accounted for 73% of respiratory deaths in this age group (Panickar et al 2005). Respiratory disease is more common in children: from a poor socioeconomic background; with a family history of respiratory disease; from an urban rather than country environment; with a school-age sibling; or with a mother who smokes during pregnancy. The highest morbidity and mortality from lower respiratory tract disease occur in the first year of life. Respiratory disease is more severe in infants with congenital heart or lung abnormalities, immunodeficiency, cystic fibrosis or chronic lung disease.
Asthma
There is considerable global variation in the prevalence rates of asthma, with the highest rates reported in America, Australasia and the United Kingdom. Much lower rates are reported in prevalence studies from Africa and Asia. Prevalence also varies considerably within countries regionally. In the 1980s to early 1990s, several cross-sectional studies from widely varying regions of the world reported an increase in the prevalence of asthma. Although many of these studies relied on self-reported symptoms, there were also reports of a parallel increase in hospitalizations and mortality rates. However, repeat cross-sectional studies over the past decade have suggested a leveling off or even a decrease in prevalance (Toelle & Marks 2005). Atopic (allergic) disease in general has increased over the past few decades and possible explanations for this rise include outdoor pollution, social deprivation/socioeconomic status, dietary factors and passive smoking (particularly maternal smoking during pregnancy). In addition, modern Westernized homes, which tend to be highly insulated (e.g. double glazing) and have increased humidity, have been recognized to be ‘dust mite-friendly’ environments. Thick pile carpets, heavily padded furniture and conventional bedding are all potential sites for dust mite activity, a known trigger for allergic reaction.
The main pathophysiological mechanism of asthma in children is inflammation within the airway, resulting in recurrent episodes of wheezing, breathlessness and cough. There is an increased responsiveness of the smooth muscle in the bronchial wall to various stimuli. Hypertrophy of the mucous glands may lead to mucus plugging. These changes cause variable airway obstruction, which may become chronic and severe.
Management
The mainstay of asthma treatment is drug therapy. There are agreed guidelines on the management of asthma (British Thoracic Society & Scottish Intercollegiate Guidelines Network (SIGN) 2003, National Asthma Education and Prevention Programme (NAEPP) 2002). The aims of therapy are to obtain optimal asthma control with few or no symptoms, undisturbed sleep, normal lung function with no limitation to daily activity and no severe, acute exacerbations. Poor asthma control has been attributed to suboptimal adherence to treatment guidelines both by physicians and families (Rabe et al 2004).
Inhalation of asthma medications provides effective topical therapy, which usually requires smaller doses and has fewer systemic effects. However, the method of drug delivery is very important and has been extensively reviewed (O′Callaghan 2000).
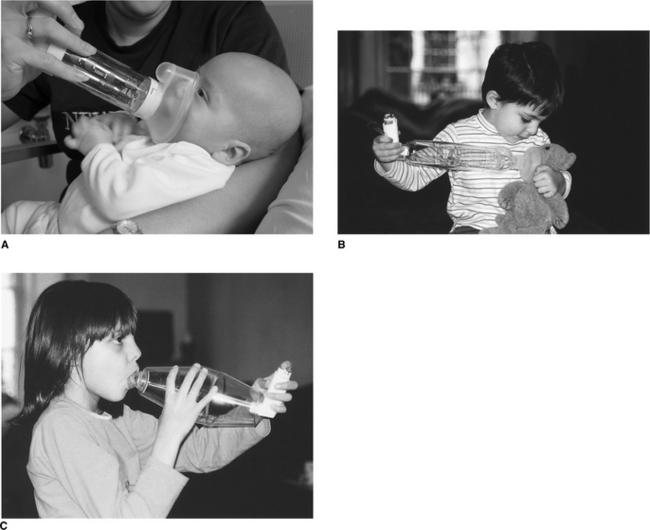
In babies, a facemask is required and should be held gently over the nose and mouth with the device held upright, at an angle greater than 45°, to ensure the valve is open. The drug can then pass effectively through the open valve to be inhaled (Fig. 10.7A & B). Once the child is older (usually from the age of 2 or 3), the spacer device can be used conventionally with a mouthpiece (Fig. 10.7C). The click of the valve opening will be heard with each breath. It should be noted that different spacer devices have been shown to deliver varying drug doses (Barry & O′Callaghan 1996).
Children with a severe asthma attack usually display signs of acute respiratory distress; they may not be able to complete a sentence in one breath or may not be able to talk, and infants show difficulty in feeding due to breathlessness. The respiratory rate is usually high (>30/minute age 5 years and above, >50/minute – age 2–5 years) and the child is tachycardic. Obvious wheezing may not necessarily be present. In life-threatening attacks, when airway obstruction in the presence of hyperinflation is severe, the airflow may be so low that wheezing is not heard, the respiratory rate is lower than expected and the chest is ‘silent’. The child may be cyanotic and is often exhausted. Children with either severe or life-threatening asthma require immediate admission to hospital. It is important to note that if nebulized bronchodilator therapy is given during an acute attack it should be oxygen driven to avoid hypoxaemia (Inwald et al 2001).