Chapter 9 Paediatric mechanical support
EPIDEMIOLOGY OF ACUTE RESPIRATORY FAILURE IN CHILDREN
Respiratory failure develops when the rate of gas exchange between the air and the blood fails to match the body’s metabolic demands. The patient therefore loses the ability to provide sufficient oxygen to the blood and develops hypoxaemia, or the patient is unable to ventilate adequately and develops hypercarbia. Epidemiologically, there is little information in children about the incidence of acute respiratory failure. Adult definitions using blood gas parameters may be appropriate for certain age groups but in others they may not be useful. For example, in infants with acute bronchiolitis, acute respiratory failure is usually defined as:
PaCO2 = 8 kPa (60 mmHg) with PaO2 = 8 kPa (60 mmHg) when using FiO2 = 0.6
However, when trying to look at large populations, in the absence of blood gases, a more pragmatic definition for acute respiratory failure is needed. For example, when using the definition of ‘acute airway management necessitating endotracheal tube intubation’ (Tasker 2000) it is possible to explore issues such as the pattern and time course of paediatric disease that have some bearing on how mechanical support should be undertaken.
Pattern of and time course of disease
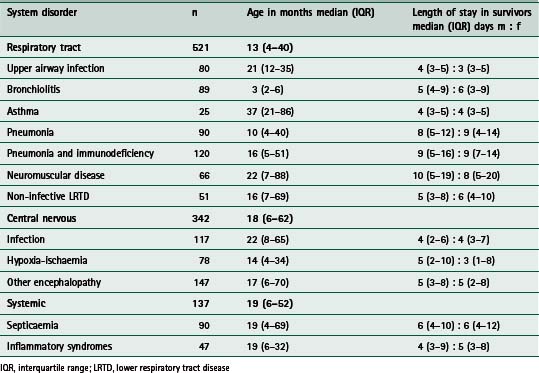
Table 9.1 summarizes a retrospective analysis of 1000 infants and children (aged older than 28 days and younger than 17 years) who required endotracheal intubation for acute respiratory failure complicating acutely acquired medical, rather than surgical, disease (Tasker 2000). The three major categories relate to the system or problem underlying respiratory failure (respiratory tract disorder, central nervous system disorder or systemic disorder) and the subcategories relate to the clinical diagnostic entities commonly encountered in intensive care. Respiratory tract problems due to infection are, not surprisingly, the most common problems seen. The time course of recovery in survivors is influenced by the site within the airways that infection has reached. This is reflected by an increase in the length of stay in the intensive care unit (ICU) with more distally affected tissues (i.e upper airway compared with lower airway). In relating such information to clinical practice one can use the expected time course to decide on an agenda for treatment or ‘care pathway’. For example, given that the expected time course for intensive care recovery in pneumonia necessitating intubation is around 8 days (interquartile range 5–12 days) one can then predict when certain targets should be met. The same applies to the other 11 distinct diagnosis-related entities. This idea will be revisited later in this section where three clinical examples are discussed.
Acute hypoxaemic respiratory failure
Acute hypoxaemic respiratory failure (AHRF) signifies respiratory failure at the more severe end of the pathophysiological spectrum, irrespective of underlying aetiology. For paediatric practice we identify this state by using diagnostic criteria that have been modified from the American-European Consensus Conference diagnostic criteria for acute respiratory distress syndrome (ARDS) (Bernard et al 1994). These criteria include:
 evidence of a severe defect in oxygenation (PaO2/FiO2 of less than 26.7 kPa, 200 mmHg) for at least 6 consecutive hours on the day of admission
evidence of a severe defect in oxygenation (PaO2/FiO2 of less than 26.7 kPa, 200 mmHg) for at least 6 consecutive hours on the day of admissionChildren meeting all the above criteria except the characteristic chest radiographic appearances of ARDS (last criterion) are described as cases of AHRF. The significance of AHRF is that it implies a certain severity of illness and risk of mortality, factors which are important when it comes to deciding which ventilatory strategy should be adopted and the use of adjunctive therapies (see section on ‘ventilation strategies for specific disease’). For example, in a prospective, epidemiological study, Peters and colleagues (1998) found that out of 850 mechanically ventilated infants and children, AHRF occurred in 118 patients (14%, 95% confidence interval (CI) 12–16%). Of these 118 patients, 52 met the criteria for ARDS (44%, 35–53%). In all 850 patients, mortality was four times higher than the mortality seen in those patients without AHRF. In the AHRF patients, mortality was three times higher for those with ARDS (Peters et al 1998). In a study from North America, the PALISI network (Pediatric Acute Lung Injury and Sepsis Investigators) reported a mortality from ARDS of 4.3% (Randolph et al 2003). This coincides with the adoption of protective modes of ventilation for this condition. Therefore, identifying these entities (i.e. AHRF and ARDS) at an early stage is important so as to institute the most appropriate method or mode of ventilation.
INDICATIONS FOR SUPPORTIVE RESPIRATORY THERAPY
Hypoxia
In infants and children, there are several methods of administering oxygen (Table 9.2). Young patients do not usually tolerate nasal catheters and cannulae. Facemasks with a reservoir and a non-rebreathing valve can be used to increase the FiO2. Alternatively, high-flow oxygen via the appropriate Venturi-valve mask can be used. Oxygen delivered via the oxygen inlet of an incubator rarely exceeds an FiO2 of 0.4. When supplemental oxygen is delivered into an tent the concentration varies depending on any leaks in the system. Regardless of the technique, it is essential that the administered oxygen is warmed and humidified. To avoid damage to the lungs, oxygen administration should be discontinued as soon as possible (as indicated by blood gas measurements). An FiO2 below 0.6 is preferred, to minimize the risk of oxygen toxicity. Reduction in the FiO2 should be carried out cautiously in a stepwise manner. To facilitate this process both the concentration and duration of oxygen therapy must be recorded accurately. A well-calibrated oxygen analyser is used to check the inspired concentration every 2 hours when using a tent, head box or when adding oxygen into an incubator. The need for monitoring PaO2 in preterm newborn infants is related to the potential for pulmonary oxygen toxicity and the danger of retrolental fibroplasia. So, in any patient, oxygen should be administered at the lowest concentration sufficient to maintain the PaO2 between 6.7 and 13.3 kPa (50–100 mmHg). Continuous measurement or monitoring of transcutaneous oxygen or pulse oximetry arterial oxygen saturation (SpO2) are essential additions to the direct, and intermittent, measurement of arterial blood gases. In some instances, supplemental oxygen may cause respiratory depression if there has been chronic respiratory failure and the patient has a hypoxic-drive to ventilation (as opposed to the normal situation where the PaCO2 is the most important factor in the control of ventilation). This phenomenon is generally uncommon in paediatric practice, but has been encountered in children with cystic fibrosis, cerebral palsy and bronchopulmonary dysplasia.
Table 9.2 Methods of oxygen administration
| Method | Maximum achievable FiO2 at 6–10 l/min of oxygen (%) |
|---|---|
| Nasopharyngeal catheter | 50 |
| Nasal prongs | 50 |
| Mask without reservoir bag | 50 |
| Mask with reservoir bag (partial rebreathing) | 70 |
| Mask with reservoir bag (non-rebreathing) | 95 |
| Venturi | 24, 28, 35, 40 |
| Incubator | 40 |
| Canopy tent | 50 |
| Head box | 95 |
FiO2, fractional inspired oxygen concentration
In addition to oxygen, some type of positive pressure may be useful in the management of hypoxia. Mask and nasal continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) increase lung compliance by recruiting additional areas of the lung for ventilation. Also, lung recruitment improves oxygenation by decreasing intrapulmonary shunt. These modes of non-invasive ventilation (NIV) are being used more frequently as the step before invasive mechanical ventilation. When invasive mechanical ventilation is used, the addition of some positive end-expiratory pressure (PEEP) is a common practice in maintaining adequate functional residual capacity. However, PEEP may adversely affect the patient lung mechanics if hyper-inflation occurs. This problem results in impaired pulmonary perfusion and further accentuates any ventilation–perfusion (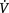 /
/ ) mismatch. Therefore PEEP above 4 cmH2O should be used judiciously if there is already regional hyperinflation, such as occurs in bronchopulmonary dysplasia (Box 9.1). In this context a strategy for treating hypoxia is outlined in Box 9.2.
) mismatch. Therefore PEEP above 4 cmH2O should be used judiciously if there is already regional hyperinflation, such as occurs in bronchopulmonary dysplasia (Box 9.1). In this context a strategy for treating hypoxia is outlined in Box 9.2.
Hypercarbia
If the patient is treated with full mechanical ventilation, the first step is to make sure that the patient is receiving an appropriate tidal volume and minute ventilation. Ventilatory system leaks and loss of a portion of the tidal volume through compressive loss in the tubing, as well as abnormalities in endotracheal tube function, are common problems that need rectifying. Having excluded mechanical factors, the other causes of hypercarbia may be related to an increase in CO2 production or an increase in dead-space ventilation. An increase in dead space may be due to excessive PEEP (particularly when there is already hyperinflation or hypovolaemia) and it may be corrected by intravenous volume to increase preload to the heart.
Endotracheal intubation
There are four absolute indications for controlling the airway by endotracheal intubation:
 maintaining the patency of the airway where problems are present or anticipated (e.g. direct airway trauma, oedema or infection)
maintaining the patency of the airway where problems are present or anticipated (e.g. direct airway trauma, oedema or infection) to protect the airway from aspiration in states of altered consciousness, where airway-protective mechanisms may be lost or impaired
to protect the airway from aspiration in states of altered consciousness, where airway-protective mechanisms may be lost or impaired to facilitate pulmonary toilet and avoid airway obstruction when there is marked atelectasis and pulmonary infection – an inadequate cough might necessitate more direct access to the airways for suctioning
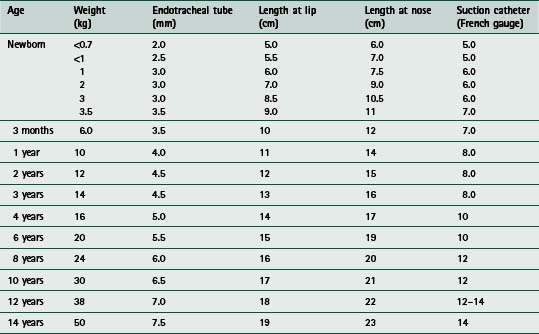
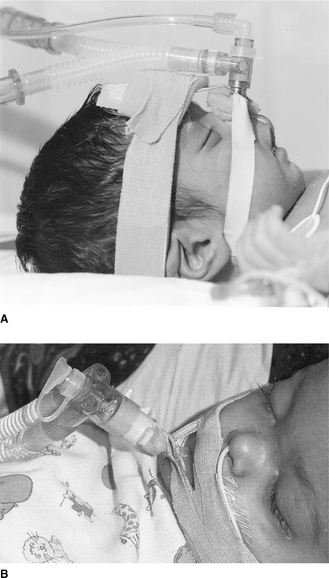
to facilitate pulmonary toilet and avoid airway obstruction when there is marked atelectasis and pulmonary infection – an inadequate cough might necessitate more direct access to the airways for suctioningIn practice, experienced staff should carry out establishing airway and respiratory support for the acutely ill child, because such patients can deteriorate rapidly, particularly at the time of inducing anaesthesia. Following preoxygenation with 100% inspired oxygen, a variety of agents are used to facilitate endotracheal intubation, including intravenous induction with drugs such as fentanyl, midazolam and suxamethonium, or inhalational induction with gases such as halothane or isoflurane. Table 9.3 provides a guide to the appropriate endotracheal tube size, length and suction catheter used in the paediatric age range and Figure 9.1 illustrates a commonly used method of endotracheal tube fixation.
MECHANICAL VENTILATION
Many of the ventilatory techniques are similar for children and adults. However, there are some differences between these two groups that are highlighted below.
General ventilatory care
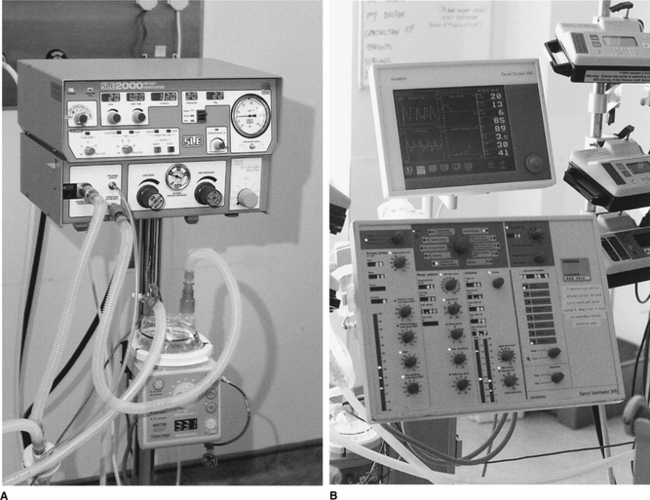
A variety of ventilators can be used in paediatric mechanical ventilation (Fig. 9.2). There are specific ventilators designed for neonates and small infants that are used mainly in neonatal units to ventilate premature babies. Fortunately most modern ventilators can be used across the whole age spectrum and they can deliver different modes of pressure control ventilation (PCV) and volume control ventilation (VCV).




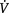 /
/ matching.
matching.