Pacemakers and Defibrillators
Cardiac pacing is the only definitive therapy for symptomatic bradycardia. Whether iatrogenic, ischemic, or intrinsic conduction system disease is present, cardiac pacing can be a temporary bridge to recovery, a backup safety therapy, or a permanent therapy, depending on the clinical scenario. What follows is a review of major topics in cardiac pacing.
Indications
Indications for cardiac pacing vary with the clinical scenario. The major determinant of need for permanent pacing is the anticipated duration of the pacing indication. For example, symptomatic bradycardia associated with a toxic ingestion of a nodal blocking drug (e.g., digitalis) can be anticipated to resolve as the drug is cleared. Temporary pacing may be indicated in the short term, but a permanent device should not be needed. Alternatively, a transient neurocardiogenic (cardioinhibitory) bradycardic episode may resolve spontaneously, and temporary pacing should not be needed. However, if episodes recur on medical therapy to the point of causing recurrent syncope, a permanent pacemaker is indicated to protect the patient from subsequent syncopal episodes.
Temporary Pacing
In the emergency department setting, transcutaneous pacing can be used as a bridge to a more definitive transvenous temporary pacing system in the setting of symptomatic bradycardia of any etiology with hemodynamic compromise.
In the critical care setting, temporary pacing can be a life saving bridge to recovery or, further, to a definitive therapy for the underlying cause of bradycardia. The indications can roughly be divided into those related to ischemia, and all other categories.
Acute myocardial infarction can be associated with bradycardia due to either sinus bradycardia, which does not require therapy unless it is causing hemodynamic compromise, or due to AV block or intraventricular block. AV block can be (a) intranodal, which is usually associated with inferoposterior infarcts (right coronary artery [RCA] 90%, left circumflex artery [LCX] 10%), manifests as first degree or Mobitz I pattern, is usually transient with benign prognosis, and rarely requires temporary pacing and almost never requires permanent pacing, or (b) infranodal, which is usually associated with anteroseptal infarcts (left anterior descending artery [LAD]), manifests as Mobitz II or third- degree block, is usually transient but may persist, and carries a poor prognosis as it signifies extensive infarction; it often requires temporary pacing and if it persists permanent pacing.
In general, “high-degree” heart block such as Mobitz type II second-degree heart block and third-degree heart block warrant temporary pacing during the acute phase of anterior (LAD territory) infarcts or inferior (RCA territory) infarcts. Further, new bifascicular block or alternating bundle branch block reflects ischemia within the interventricular septum and warrants temporary pacing as a backup in case of progression to complete heart block. Refractory bradycardia in the setting of an infarct in any territory necessitates temporary pacing.
In the absence of an acute myocardial infarction, symptomatic bradycardia with or without AV dissociation and third-degree AV block with ventricular escape warrant temporary pacing. Backup pacing indications include temporary ventricular pacing during right heart catheterization in the setting of preexisting left bundle branch block (LBBB), new bundle branch block or AV block in the setting of endocarditis, and essential pharmacologic therapies that may induce or exacerbate bradycardia.
Temporary pacing systems with temporary epicardial atrial and ventricular wires are routinely used in the setting of open heart surgery. These systems are used to optimize cardiac output coming off cardiopulmonary bypass, and subsequently as a backup system in case AV nodal conduction block occurs postoperatively, especially in the setting of valvular heart surgery.
The indications for permanent pacing are listed in detail in the ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). This document is summarized in Table 31.1.
TABLE
31.1 Indications for Permanent Pacemakers


AV atrioventricular; MI, myocardial infarction; SND, sinus node dysfunction; VT, ventricular tachycardia; LV left ventricular; EPS, electrophysiology study; CRT, cardiac resynchronization therapy; NYHA, New York Heart Association; HV Histo ventricular conduction time; DCM, dilated cardiomyopathy; LVEF, left ventricular ejection fraction; HOCM, hypertrophic obstructive cardiomyopathy.
From Writing Committee Members Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Circulation. 2008;117:2820-2840, with permission.
Device Features
Single-chamber devices that only pace the ventricle or atrium have fallen by the wayside in favor of more sophisticated atrioventricular pacing devices that have the ability to track the sinus node rate when appropriate and pace the ventricle after a set delay. They also switch modes to ventricular backup pacing when the atrial signal falls outside of set parameters, as in paroxysmal atrial fibrillation and sick sinus syndrome. Further, cardiac resynchronization therapy (CRT) with biventricular pacing has a significant beneficial role in patients with symptomatic heart failure (New York Heart Association [NYHA] Class III and ambulatory Class IV) and evidence of left ventricular dysynchrony (EF ≤ 35% and wide QRS duration ≥120 milliseconds). Despite the predominance of dual-chamber pacemakers, there are increasing data suggesting that right ventricular stimulation increases the incidence of heart failure, hospitalization, and death in various patient subsets. However, if the ventricle needs to be stimulated, the vast majority of patients tolerate right ventricular stimulation, as dual-chamber stimulation is preferred over sole ventricular stimulation. Figure 31.1 shows a schematic of pacemaker timing cycles.
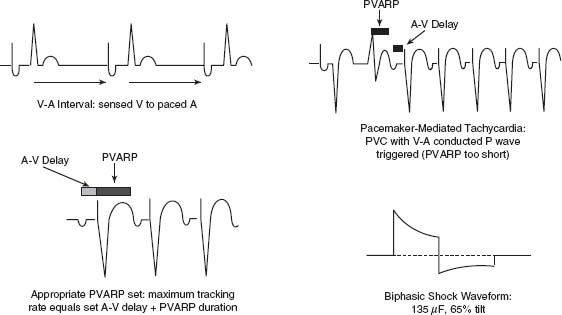
FIGURE 31.1 Schematic of important device timing cycles and impulses.
Rate-Adaptive Pacing
A variety of methods have been employed to allow for implantable pacemakers to increase their pacing rate in the setting of metabolic demand for increased cardiac output. The most commonly employed methods include activity sensors (vibration, acceleration), or minute ventilation sensors. Other sensors include peak endocardial oxygen sensors and right ventricle (RV) impedance-based sensors, which have the advantages of responding to nonexertional stimuli (emotions). These techniques utilize vibration, acceleration, minute ventilation, or other measurements as a surrogate for increased metabolic demand for oxygen delivery. In patients with chronotropic incompetence, or the inability to increase cardiac output in response to exercise, rate-adaptive devices can utilize these surrogates to increase the pacing rate and therefore increase cardiac output.
There are advantages and disadvantages to each type of sensor system. Vibration sensors and accelerometers provide an almost immediate rise in rate and therefore cardiac output when they detect activity. However, they can be “fooled” by stimuli external to the patient that mimics patient activity (i.e., turbulence during flight, etc.). The accelerometer tends to respond more specifically to patient activity than does the motion sensor. The advantage of the minute ventilation sensor is that it responds specifically to the patient’s respiratory rate—a parameter that is controlled by the brainstem. Although this parameter perhaps more reliably reflects the degree of patient exertion, it tends to lag behind the initiation of strenuous activity. Dual-sensor systems that utilize data collected from more than one sensor modality may actually be best suited for effective rate-adaptive pacing.
Mode Switching
Another feature of dual-chamber cardiac pacemakers that allows the devices to respond to changes in the physiology of the patient is mode switching. Mode switching is the ability of the device to revert to a separate, backup pacing mode in the event that the primary pacing mode no longer best serves the patient’s pacing need. For example, in a patient with AV nodal block, a dual-chamber device may be programmed to sense or track the patient’s intrinsic sinoatrial rate and to pace the ventricle after a set delay within the range of 60 to 120 beats/min (bpm). If the atria begin to fibrillate, the sensed atrial rate would exceed the rate parameter and the device would switch modes to a backup ventricular-only mode with a set rate sufficient to prevent hemodynamic compromise. If the patient reverted to sinus rhythm subsequently, the device would recognize the atrial rate back within the set parameter range and switch back to the primary mode, tracking the atrium and pacing the ventricle. Mode switching allows for the maximum responsiveness to the patient’s intrinsic rhythm. These devices are most commonly programmed DDDR and revert to VVIR during periods of high atrial rates.
Other Programmable Features
Modern pacemakers now include a myriad of programmable features to better match the patient’s physiologic status. They can be programmed to pace at a lower “sleep” rate during typical sleeping hours, with absence of strenuous activity confirmed by the devices metabolic sensing system. Programmable pulse width and output allow the programmer to optimize the impulse specifications to ensure capture while preserving battery power. Diagnostic information and event data including mode-switching data can be stored and retrieved later to assess for the presence and prevalence of atrial arrhythmias and other events. Atrial and ventricular electrograms can be obtained and stored. Device status data can also be retrieved, including battery usage and projected battery life given current settings.
Leads
The pacing leads conduct the electrical pacing impulse to the myocardium, and conduct the intrinsic electrical activity of the myocardium to the sense amplifiers within the device. Unipolar leads have a single electrode at their tip, and therefore they direct current from their tip to the can of the device through the patient’s tissues, or vice versa. For this reason, problems such as pectoral, intercostal, or diaphragmatic stimulation are more likely to occur, particularly in implants requiring higher outputs to capture the ventricle. Bipolar leads have two electrodes with close proximity at their tip and direct current proximal to distal or distal to proximal over much smaller distances. These leads can achieve capture of the myocardium with lower output energies and thus are more efficient. They are capable of unipolar function as well, but with the same limitations as standard unipolar leads. Of note, bipolar leads are by necessity larger and stiffer than unipolar leads, and have been historically more prone to mechanical failures than unipolar leads.
Coronary sinus leads are small, highly flexible unipolar or bipolar leads. They can be directed from the right atrium via the coronary sinus into a branch cardiac vein for the purpose of pacing the left ventricle in synchrony with the RV in patients with ventricular dysfunction and delayed intraventricular conduction, usually manifest as a LBBB.
Epicardial leads can be placed surgically using minimally invasive techniques or during open heart surgery for another indication and subsequently utilized instead of transvenous leads for standard pacing or more commonly for CRT (biventricular pacing). Often two leads are placed at the time of surgery and one of the two is subsequently utilized for biventricular pacing, depending on the thresholds and pacing characteristics of each at the time of device implant.
A variety of fixation techniques are utilized to maintain the contact of the lead tip with the myocardium. Active fixation leads employ a fixed extended or retractable screw to engage the myocardium. These systems allow for better localization of the lead tip at the desired site of implantation during deployment of the fixation helix. Passive fixation systems utilize plastic projections near the distal electrode that entrap in the trabeculations of the right ventricle or the right atrial appendage to maintain the position of the lead. As lead implants “mature” over time, pacing thresholds tend first to rise due to inflammation and then improve as healing continues and the inflammation resolves. Most leads have a small amount of steroid impregnated at the lead tip that reduces the size of the fibrotic tissue capsule and reduces the chronic thresholds.
Basic Concepts of Impulses and Timing
Following is a review of some basic concepts in pacemaker theory that are central to an understanding of the clinical application of pacing technology.
Stimulation threshold: The minimum amount of electrical energy that consistently produces a cardiac depolarization. The energy is a combination of voltage and pulse duration. It can be expressed in terms of amplitude (milliamperes or volts), pulse duration (milliseconds), charge (microcoulombs), or energy (microjoules).
Voltage output: The amount of voltage being delivered to the heart every time the pacemaker emits a stimulus. It is expressed in volts.
Pulse width (or pulse duration): The length in milliseconds the voltage is delivered to the heart.
Strength-duration curve: The hyperbolic relationship between the voltage output and the pulse width that defines the stimulation threshold (Fig. 31.2).
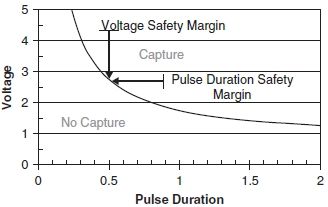
FIGURE 31.2 The strength–duration curve.
Sensing: Sensing occurs when the electrical wave front through the myocardium passes directly underneath the electrode
Atrial sensitivity: A programmed parameter that defines the largest signal that will be ignored by the device and thus determines which signals are detected by the pacemaker or implantable cardioverter/defibrillator (ICD) in the atrial channel. Atrial sensing in the dual-chamber pacing mode, DDD, will inhibit the atrial stimulus which would occur at the end of the atrial escape interval (V-to-A interval), initiate the AV interval, and trigger the ventricular output at the end of the AV interval.
Ventricular sensitivity: A programmed parameter that defines the largest signal that will be ignored by the device and thus determines which signals are detected by the pacemaker or ICD in the ventricular channel. Ventricular sensing in the dual-chamber pacing mode, DDD, will inhibit both atrial and ventricular stimuli that were scheduled to be output at the end of the atrial escape interval (atrial) or AV interval (ventricle) and initiate a new atrial escape interval (V-to-A interval).
Atrial oversensing: Sensing on the atrial channel that occurs due to signals on the atrial lead either related to signals originating outside the atrium, such as far-field ventricular signals, myopotentials from the pectoralis major muscle or diaphragm, or from noise originating from a dysfunctional lead (insulation or conductor fractures or a loose set screw). Depending on the mode of pacing, atrial oversensing will either inhibit or trigger atrial and/or ventricular stimuli.
Ventricular oversensing: Sensing on the ventricular channel that occurs due to signals on the ventricular lead either relating to signals originating outside the ventricle, such as myopotentials from the pectoralis major muscle in a unipolar lead system or from lead dysfunction secondary to insulation or conductor fracture or loose set screws. Sometimes the ventricular channel will oversense the atrial paced output and inhibit the ventricular output. This is called crosstalk inhibition and is usually prevented by a blanking of the ventricular sensing amplifier during the atrial paced outputs.
Chronotropic competence: The ability to match cardiac output to the metabolic needs of the body by appropriate modification of the heart rate.
Minimum rate: Also called the escape rate, this is the slowest rate at which the pacemaker will allow the heart to beat. The minimum paced rate is calculated by the ventricular paced or sensed event to atrial paced output interval plus the programmed A–V delay measured in milliseconds and converted to rate by dividing 60,000 by that sum.
V–A interval: Also called the atrial escape interval, this is calculated by subtracting the paced AV interval from the minimum rate interval. It is initiated by a paced or sensed ventricular event and concludes with a paced atrial event or is interrupted by either an atrial or ventricular sensed event.
A–V delay: This programmed interval is initiated by an atrial sensed or paced event and is terminated with a ventricular paced stimulus unless interrupted by a ventricular sensed event (either a conducted beat through the AV node or a premature ventricular beat). Often AV delays initiated by sensed atrial events are programmed to be shorter than AV delays initiated by atrial paced events.
Upper rate limit: The fastest rate at which the ventricular channel can track intrinsic P waves or, in the case of rate-adaptive pacing on the basis of a sensor, the fastest rate at which the ventricular channel can track the sensor rate algorithm. The atrial tracking or upper rate limit is constrained by dividing 60,000 by the sum of the sensed AV delay and the postventricular atrial refractory period (PVARP).
PVARP: This is the Post-Ventricular Atrial Refractory Period. The PVARP is the timeframe during which the atrial channel is refractory after either a paced or sensed (R wave) ventricular event. Its purpose is to prevent atrial sensing and tracking of any V–A (retrograde) conduction of ventricular events to the atrium that would trigger a pacemaker-mediated tachycardia (see later).
Programming
Device programming has become more complex as dual- chamber pacing systems have become ubiquitous and biventricular pacing is becoming common place. It is important to note that the basic parameters discussed above can usually be derived with caliper measurements of the intervals observed on a 12-lead electrocardiogram (ECG) or rhythm strip. Following is a concise review of programming codes and timing cycles that provide the underpinnings of device programming.
Codes
A standard coding system has been adopted to delineate the basic settings of the device as follows: The first designation is the chamber paced, the second designation is the chamber sensed, the third designation is the device response to a sensed event, and the final designation reflects the rate-adaptive status of the device. There is a fifth position in the code that was originally used to indicate the antitachycardia response that the device will provide during a tachycardia, but now indicates whether multisite pacing is present or not. Table 31.2 reviews the mode codes in detail.
TABLE
31.2 Mode Codes for Cardiac Pacemakers

As an example, a DDIR pacemaker can pace in both the atrium and the ventricle, and sense activity in both the atrium and the ventricle. Further, it will inhibit upon sensing intrinsic activity, and it has rate-adaptive functionality as well. A VOO device will pace the ventricle asynchronously, without sensing intrinsic activity.
Timing Cycles
When a dual-chamber device paces the atrium, the ventricular channel is blanked for a period of 20 to 40 milliseconds as a safety feature to prevent inhibition of the ventricular channel by far-field (ventricular) sensing of the atrial paced output. The blanking period prevents “crosstalk inhibition,” which could cause, in patients with complete lack of AV conduction, a string of atrial paced events and ventricular asystole. After the blanking period, the ventricular channel is open to sensed events. Typically, the first 100 milliseconds is the safety alert period. If, during this alert period a sensed event occurs, then the AV interval is abbreviated, usually to 120 milliseconds. This abbreviated AV delay is designed to prevent pacing during the vulnerable period of the ventricle, for instance, when the sensed event is caused by a premature ventricular depolarization. During the remainder of the AV delay (after the blanking and safety alert period), any sensed ventricular event will cause the ventricular output to be inhibited and reinitiate the atrial escape interval. If by the end of the programmed AV interval no event has been sensed, the device will pace the ventricle. After every paced or sensed ventricular event, a PVARP is initiated. During this period, the atrial channel is refractory to detecting atrial activity. The purpose of the PVARP is to prevent detection of atrial activity produced by retrograde conduction through the AV node. Without making the atrium refractory to retrograde atrial events (V-to-A conducted beats) an endless loop cycle can be set up that continues until the retrograde conduction fails. This endless loop tachycardia is one of several types of pacemaker-mediated tachycardia (see Fig. 31.1 for a schematic of pacemaker timing cycles in comparison to the surface ECG).
Diagnostics
Modern pacing devices are capable of storing tremendous amounts of data and reporting data in a variety of usable formats. Following is a brief review of device diagnostics and their applications.
Histograms. Histograms are a statistical report of a parameter describing the relative frequency of an event relative to time, heart rate, or another parameter. Histograms do not correlate symptoms to specific events, and from them one can only infer cause and effect (Fig. 31.3).
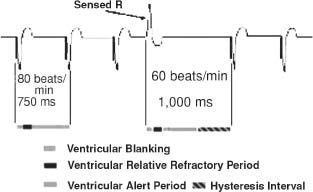
FIGURE 31.3 Histogram: ventricular hysteresis.



