Fig. 1.1
The three zones of the neck. Zone I is located below the cricoid cartilage. Zone II is located between the cricoid cartilage and the angle of the mandible. Zone III is located above the angle of the mandible. Injury to zone I occurs in about 18 % of patients with neck trauma, zone II injuries occur in 47 %, and zone III injuries occur in 19 %
Table 1.1
Hard and soft signs of vascular injury
Hard signs | Soft signs |
|---|---|
Shock/refractory hypotension | Non-pulsatile bleeding |
Pulsatile bleeding | Stable hematoma |
Audible bruit | Nerve injury |
Enlarging hematoma | Unequal blood pressures/pulse exam |
Loss of pulse with neurologic deficit | Proximity of injury tract |
1.2.2 Blunt Injury
The overall incidence of blunt cerebrovascular injury (BCVI) has been universally reported as less than 1 % of all admissions for blunt trauma, but this relatively small population of patients has stroke rates ranging from 25 to 58 % and mortality rates of 31–59 % [4–6]. The incidence of BCVI is 0.19–0.67 % for unscreened populations and 0.6–1.07 % for screened populations [7]. The recognition and treatment of BCVI has evolved dramatically over the past two decades. As imaging technology has improved with respect to both image quality and acquisition times, CT has become a fundamental diagnostic tool in blunt trauma evaluation. A current grading scale for blunt cervical vessel injury is presented in Table 1.2.
Table 1.2
Blunt cerebrovascular injury grading scale
Grade | Angiographic findings | Stroke risk (%) | Mortality (%) |
|---|---|---|---|
I | Luminal irregularity, dissection, or intramural hematoma with <25 % luminal narrowing | 3 | 11 |
II | Luminal irregularity, dissection, or intramural hematoma with ≥25 % luminal narrowing | 11 | 11 |
III | Pseudoaneurysm | 33 | 11 |
IV | Vessel occlusion | 44 | 22 |
V | Vessel transaction | 100 | 100 |
1.2.3 Evaluation
Computed tomography is the workhorse of trauma evaluation and should be the initial diagnostic step in patients with penetrating neck injuries but no hard signs of vascular injury. Computed tomographic angiography (CTA) has a 90 % sensitivity and 100 % specificity for vascular injuries that require treatment [9, 10]. Occult injuries (intimal flaps, dissections, pseudoaneurysms) identified during the evaluation for penetrating cervical injury should be considered for management similar to those caused by blunt trauma. Table 1.3 summarizes the evaluation criterion from three major investigator groups that should trigger CTA in the evaluation of blunt cervical vessel injuries. CTA evaluation should always include the head and neck, and if zone I injuries are suspected, the aortic arch should also be included. This can be completed with a single contrast bolus in a well-timed exam.
Table 1.3
Screening criteria for blunt cerebrovascular injury
Denver criteriaa | Memphis criteriab | Modified Biffl criteriac |
|---|---|---|
Arterial hemorrhage | Neurologic exam not explained by imaging | GCS <6 |
Expanding hematoma | Horner’s syndrome | |
Cervical bruit | Neck soft tissue injury (seat-belt sign, hanging, or hematoma) | |
Neurologic exam inconsistent with head CT findings | ||
New stroke on follow-up imaging | ||
New focal neurologic deficit | ||
Le Fort II or III fracture pattern | Le Fort II or III fractures | Le Fort II or III fractures |
Basilar skull fracture with involvement of carotid canal | Basilar skull fracture with carotid canal involvement | Petrous fracture Diffuse axonal injury |
Diffuse axonal injury with GCS <6 | Cervical spine fracture | |
Cervical spine fracture | ||
Near-hanging with anoxic brain injury |
1.2.4 Carotid Artery Injuries
1.2.4.1 Treatment of Blunt Injuries I–IV
The mainstay of treatment for BCVI grades I–IV is antithrombotic therapy, traditionally with heparin infusion followed by warfarin therapy [5]. Because of the concern for full anticoagulation of the recent trauma patient, others have investigated the use of antiplatelet therapy alone [11, 13, 14]. Some have found lower stroke rates with heparin compared to antiplatelet therapy [6]. Unfortunately, there are no prospective head-to-head trials of heparin versus antiplatelet therapy; therefore, a prudent protocol includes heparin therapy and transition to warfarin if an absolute contraindication to systemic anticoagulation does not exist. If full anticoagulation is not able to be completed, antiplatelet therapy should be initiated. These lesions all require follow-up imaging with either CTA or catheter-based angiography anywhere from 1 to 3 months post-injury. Edwards et al. found that at 3 months, one can expect 72 % of grade I injuries to be completely healed [15]. Grade II injuries are fairly evenly distributed: 33 % are improved, 33 % are stable, and 33 % progress [15]. Grade III injuries tend to either remain unchanged or enlarge but rarely resolve [15]. These lesions typically have a low risk of rupture but can be a source of distal embolic events or thrombosis, and as such, continued anticoagulation is recommended [16, 17].
1.2.4.2 Treatment of Penetrating Injuries and Grade V Blunt Injuries
The management of grade V blunt injuries (complete transaction) and penetrating injuries is a much more complex decision tree. In essence, they may be treated as synonymous injuries. Penetrating injuries in zone II should be operatively explored and repaired surgically. This can be accomplished via a cervical incision. The external jugular and facial veins may be ligated with little concern. Injuries to the internal jugular vein may be primarily repaired but can be ligated in emergency conditions if necessary. Whenever surgical intervention is undertaken, at least one leg must be prepped to allow for vein harvesting for patch or interposition conduit harvesting. Saphenous vein patch can be used to repair partial injuries and should be harvested from the groin rather than the ankle. Alternatively, some have used bovine pericardium if no vein is present, but this carries increased risk of infection in a contaminated field. Injuries due to iatrogenic cannulation or from stab wounds can often be repaired primarily; ballistic injuries most often require segmental resection and interposition grafting. The saphenous vein offers a good size match for the internal carotid in these cases and in some cases for the distal common carotid. If size mismatch is an issue, some have proposed the use of the superficial femoral artery with interposition polytetrafluoroethylene (PTFE) grafting in the superficial femoral artery (SFA) harvest location [4]. This allows for autologous reconstruction in the contaminated field and prosthetic reconstruction in the clean harvest bed. The patient should always be fully heparinized prior to any clamping of the carotid system; temporary shunts can be used at the discretion of the operative surgeon. Simple ligation of the carotid artery has significant consequences and results in nearly 45 % mortality [1]. Because of this, it should be reserved only for those injuries at the base of the skull that are not amenable to reconstruction or when complete transection with thrombosis is already present without resulting neurologic incident.
Penetrating carotid artery injuries in zone I and III present a much more complex problem. Proximal and distal control can be a significant issue or require much more morbidity with jaw dislocation, mandibulotomy, median sternotomy, or trapdoor incisions. Because of this, endovascular techniques have increased in popularity for control of these injuries. These techniques have the added benefit of being able to be completed under local anesthesia, allowing for continuous neurologic monitoring. Multiple groups have shown low-risk profiles with the use of covered stents for the treatment of hemodynamically significant dissection, pseudoaneurysms, partial transections, and other injuries to the carotid vessels in these zones [18–25].
1.2.5 Vertebral Artery Injuries
Vertebral artery injuries (VAIs) are quite rare, with average incidence of 0.20–0.77 % among all trauma admissions [5, 6]. Other than the first segment of the vertebral artery, V1, the remaining portions of this vessel are difficult to access from open surgical approaches due to the significant bony protection. The vertebral artery is most often injured from cervical spine transverse process fractures, subluxations, or penetrating injuries to the back of the neck [26]. Catheter-based selective angiography remains the gold standard, but as with carotid injury, CTA has gained popularity due to its ease of access and rapid initiation. CTA has been shown to have a sensitivity and specificity of 40–60 % and 90–97 %, respectively [27].
1.2.5.1 Treatment of Blunt Injuries
As with blunt carotid injuries, the mainstay of blunt vertebral injuries is systemic anticoagulation, unless complete transection with extravasation is noted. Studies from the past decade have shown a reduced neurologic incident rate from 20 to 35 % with no anticoagulation to 0–14 % with heparin therapy [7, 28]. Again, as with carotid blunt injury, if systemic anticoagulation cannot be administered, antiplatelet therapy should be given.
1.2.5.2 Treatment of Penetrating Injuries and Blunt Transection Injuries
Because of the difficult surgical access to the vertebral vessel, endovascular techniques have become a first approach to the treatment of penetrating and blunt transection injuries. Selective angiography and crossing of the lesion can allow for proximal and distal coil embolization in most patients. Even in cases of complete transection, crossing of this lesion can be successful, allowing endovascular treatment. Up to 50 % of the time, selective angiography will reveal that the vessel has already thrombosed, and thus no further therapy is needed [29]. There have been rare reports of covered stent graft placement in the vertebral system, but this is not routinely performed [7].
If endovascular techniques are not available or do not succeed in controlling the bleeding, open operative ligation can be completed with an expected stroke rate of 3–5 % [30]. The most straightforward approach involves isolation of the V1 segment of the vertebral artery and ligation at this point, with packing of the wound to assist in retrograde and collateral back bleeding. This portion of the vertebral artery can be obtained through the same exposure as the carotid artery. The sternocleidomastoid muscle attachments to the sternum and clavicle are taken down, the scalene fat pad is mobilized, and the anterior scalene muscle is divided with care not to injure the phrenic nerve. At this point, the subclavian artery and origin of the vertebral artery can be dissected and ligated to control bleeding.
1.3 Thoracic Vascular Injuries
Thoracic vascular injuries carry with them a high degree of lethality. By best estimates, thoracic aortic injuries result in 50–80 % in-the-field mortality. Those patients that do survive to hospital evaluation require rapid and accurate diagnosis and intervention. In most urban environments, upwards of 90 % of these injuries are a result of penetrating mechanisms [31]. In contrast, in more isolated and rural setting, the majority are due to blunt aortic transections from deceleration injuries. Regardless, the initial evaluation should consist of chest radiographs, and in the hemodynamically stable patient, CTA plays a key role in the radiographic survey. Unstable patients with great vessel or thoracic aortic injury often undergo emergency room thoracotomy. Once the chest has been opened, aortic clamping with further resuscitation and transport to the operating theater for definitive repair may be undertaken. This section will focus on the aortic arch, its branches, and the descending thoracic aorta. Both penetrating and blunt injuries will be discussed. The rising use of endovascular techniques for the management of these injuries will be of specific discussion.
1.3.1 Ascending Aorta and Transverse Arch
Injury to the ascending aorta and transverse arch is most often associated with penetrating mechanisms but can be seen with severe blunt force injury. There is exceedingly high in-the-field mortality with these most proximal aortic injuries. Clinical and radiographic signs include cardiac tamponade, widened mediastinum, and apical capping. These injuries should be approached with an open technique via a median sternotomy utilizing cardiopulmonary bypass (Fig. 1.2). Depending on the extent of the injury, primary repair or interposition grafting may be necessitated. Posthospital survival rate depends greatly on clinical presentation, comorbid conditions, and other associated injuries [32, 33].
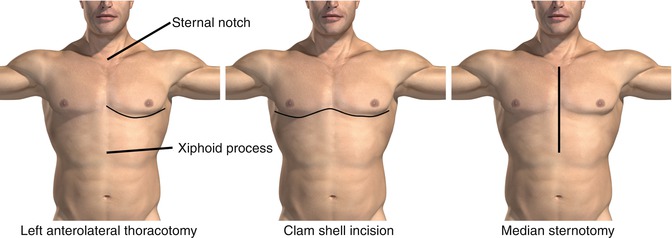

Fig. 1.2
Three major incisions used for access to mediastinal structures. The left anterolateral thoracotomy permits rapid access to mediastinal and left chest contents and is particularly useful for cross clamping the aorta after major trauma with hemorrhage. The clamshell incision permits rapid access to the entire chest and can be used by extending the initial incision made for a left anterolateral thoracotomy if a major right chest trauma is suspected. Finally, a median sternotomy permits access to the mediastinum. A right or left cervical extension can be utilized for access to the subclavian and carotid vessels
1.3.2 Innominate Artery
Innominate artery injuries can result from blunt, penetrating, and iatrogenic sources. The most common iatrogenic mechanism is with central line misadventures. Classically, open repair via a median sternotomy with a right cervical extension was the approach of choice to repair of these injuries. Primary repair or short segment interposition grafts can be utilized to repair these injuries. Because of the potential morbidity of these procedures, endovascular techniques have gained popularity in recent years. There have been multiple reports of cover stent graft implantation for the management of these injuries [34–37]. As a result of this growing body of literature, consideration for covered stent graft implantation should be given. Because of the immediate proximity to the cerebrovascular circulation, systemic anticoagulation during cannulation and deployment should be complete, and post-implant treatment with antiplatelet agents should be strongly considered.
1.3.3 Proximal Left Carotid Artery
Surgical exposure for intervention on the proximal left common carotid artery is a mirror exposure of that of the innominate artery. Median sternotomy with left cervical extension is utilized (Fig. 1.2). While primary repair can be completed in some cases, interposition grafting is the preferred method of repair in an open intervention. As with the other arch vessels, management has shifted to endovascular techniques in recent years. The implantation of covered stent grafts has gained significant popularity [38–41]. As with innominate artery stent implantation, full heparinization during the procedure is essential, and post-implantation antiplatelet therapy is strongly advised. Additionally, the use of distal embolic protection is strongly advised when carotid artery interventions are undertaken [42].
1.3.4 Left Subclavian Artery Injuries
Surgical exposure of the left subclavian artery can be difficult and may require both a supraclavicular and anterolateral thoracotomy (Fig. 1.3). Some have connected these with a median sternotomy for full exposure, but this incision is wrought with complications and can be difficult to complete if not experienced in this technique. Clavicle resection may also be needed to gain complete control of these injuries. Because of the complex surgical exposure, interest in endovascular approaches has gained significant momentum. As with other arch vessels, the use of covered stent grafts has shown promising results with injuries in this location [43–46]. These injuries may be approached from either the ipsilateral brachial or either femoral access points depending on the associated bony extremity injuries.
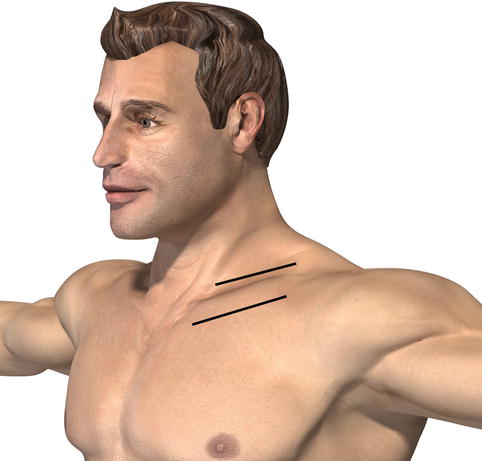

Fig. 1.3
Subclavian exposure. A supraclavicular and infraclavicular incision can be used for access to the subclavian artery. A median sternotomy may be necessary to achieve proximal control, particularly in trauma
1.3.5 Descending Thoracic Aorta
Most penetrating injuries of the descending aorta result in in-the-field mortality or significant hemodynamic instability prompting emergent thoracotomy. Once identified, these injuries can be repaired with traditional open techniques, including primary repair or interposition graft. With improved prehospital care and automotive safety, survival to hospital presentation with blunt aortic injury is increasingly more common. As a result, this section will deal mostly with the diagnosis and treatment associated with blunt aortic injury (BAI) and traumatic aortic disruption. The exact incidence is not truly known, but the EAST Practice Guideline of 2,000 estimated that BAI accounted for roughly 8,000 deaths annually in the United States [47]. There are multiple theories on how BAI develops, including acute intraluminal pressure spikes, stretch and shear strain, and bony compression, but a description of these in detail is beyond the scope of this chapter. Regardless of the mechanism, BAI typically results in disruption near the aortopulmonary ligament at the embryologic remnant of the ductus arteriosus. Widened mediastinum on chest radiograph is the classic finding, but rib 1–3 fracture, sternal fracture, clavicle or scapular fractures, or apical capping should all raise the index of suspicion. If hemodynamically stable, CTA is essential in case planning, especially for endovascular approaches. If endovascular repair is chosen, CTA through the pelvis is ideal to assess the iliofemoral system for large-bore introducer cannulation needed to deploy thoracic endografts. Once a BAI has been confirmed, strict blood pressure and heart rate control should be undertaken until the diagnostic work-up is completed and repair has occurred. Fabian et al. have shown that strict impulse control (blood pressure and heart rate) results in decreased morbidity [48, 49]. In summary, they recommend a goal systolic blood pressure <100 mmHg, a goal mean arterial pressure <80 mmHg, and a goal heart rate of <100 BPM [48, 49]. This can be accomplished with esmolol infusion or PRN labetalol with or without the aid of nitroglycerine infusion to assist in vasodilation. Once impulse control has been established and provided that hemodynamic stability persists, the patient can receive the remainder of the trauma evaluation. Other immediately life-threatening injuries can be dealt with first, and if no other emergent operative indications exist, repair of the BAI can be undertaken.
Historically, these injuries have been treated with open left lateral thoracotomy with or without cardiopulmonary bypass and hypothermic circulator arrest (Fig. 1.2). Interposition grafting is almost universally needed, and primary repair is general not advisable. A complete description of the operative exposure and repair is beyond the scope of this chapter; however, as with any thoracic aortic surgery, subsequent paraplegia from spinal ischemia is a feared consequence [50–52]. With aggressive and protocolized approaches to spinal cord protection, this often fatal complication can be prevented in many patients [53–57]. As with the other vessels described in this section, endovascular techniques are a rising component in the management of BAI. The use of thoracic endovascular aneurysm repair (TEVAR) devices for the management of BAI is becoming increasingly common [58–62]. Additionally, others have reported the use of abdominal aortic extension cuff in the thoracic aorta for the treatment of BAI [60]. As with any off-label use, the merits of this technique must be weighed against the benefits and risks of a non-FDA-approved application of such devices. There has been some concern about the long-term implication of device implantation in a typically young trauma patient population. Additionally, concern has been raised about the ability to maintain long-term follow-up in this patient population [63]. Despite these concerns, TEVAR for BAI has been recommended as the treatment of choice by many [60, 62]. The current generation of TEVAR devices is approved for vessel diameters of 16 mm and larger; because of this, the use in very young and pediatric populations is generally not advised due to the absolute vessel size and long-term consequences of future growth. In these extenuating circumstances, open repair is advised.
1.4 Abdominal and Pelvic Vascular Injuries
Vascular injuries within the abdominal and pelvic cavities can be difficult to manage as a result of concomitant injury to both hollow and solid organs. Traditionally, many intra-abdominal vascular injuries have been addressed in an open fashion as there is often an indication for exploration due to these other associated injuries. However, there does exist a subset of abdominopelvic vascular injuries that can be addressed via endovascular approaches. Abdominal vascular injuries account for 30 % of all vascular trauma, and about 90 % are caused by penetrating trauma [64]. In patients undergoing exploratory laparotomy, it is estimated that the incidence of concomitant vascular injury is 14 % for gunshot wounds, 10 % for stab wounds, and 3 % for blunt injuries [65, 66]. Most patients with abdominopelvic vascular injuries present with hard signs of vascular injury or hemodynamic instability due to the large potential space for bleeding and other associated injuries that typically accompany. Because of this, most of these injuries are directly discovered at the time of exploratory laparotomy, and little work-up is typically undertaken. With smaller, more distal branch vessel injury, patients may undergo abdominopelvic CT, allowing for a more thorough evaluation.
1.4.1 Abdominal Aorta Injuries
Abdominal aortic injuries are almost uniformly penetrating in nature. Blunt injury to the abdominal aorta accounts for 0.04 % of trauma admissions [67]. Gross contamination is common in patients with penetrating trauma, as 93 % of patients have other associated intra-abdominal injuries including the small bowel (45 %), colon (30 %), and liver (28 %) [68]. The use of prosthetic graft has always been an area of controversy. We recommend the use of autogenous tissue for reconstruction whenever possible for these situations. Enteric spillage should rapidly be controlled and the peritoneal cavity irrigated in all cases however. Prosthetic grafts may be necessary for large or complex repairs, and bowel content spillage is not considered an absolute contraindication by some [69].
There have been case reports of the management of abdominal aortic injuries using endovascular techniques, but the current generation of devices is limited in their utility for injuries in this location. Pseudoaneurysms, aortocaval fistulae, and infrarenal aortic dissections have all been treated successfully with stent grafts [67, 70–72]. The limiting factor preventing more widespread application of endovascular techniques in repair of the abdominal aorta is branch graft technology. Until fenestrated and branch graft technology becomes more available, the use of endovascular grafts will have a definitive role in selected cases of infrarenal aortic injury.
1.4.2 Celiac Artery Injuries
Celiac artery injuries are often accompanied by other vascular injuries and most often the result of penetrating injuries. Ligation is generally well tolerated for celiac and celiac branch vessel injury. The portal vein and gastroduodenal artery are often adequate blood supply for liver parenchyma should the common hepatic artery require ligation; however, late bile duct strictures can be encountered if distal hepatic branches are ligated. Because of the rich collateral network in this location, catheter-directed embolization is also a viable technique. Additionally, there have been a few reports of endovascular covered stent grafts, though tortuosity is a complicating factor. Celiac artery injuries are considered a marker of more severe trauma, and published mortality rates range from 38 to 75 % [73]. Many of these mortalities are related to other associated injuries and not directly attributable to the celiac disruption.
1.4.3 Superior Mesenteric Artery (SMA) Injuries
For traumatic injury purposes, the SMA can be divided into two segments, marked by the takeoff of the middle colic artery. Proximal to this, ligation results in significant bowel ischemia from the ligament of Treitz to and including the right hemi-colon. Distal to this, ligation can result in partial bowel ischemia, and segmental resection may be needed. In the unstable patient, as an alternative to ligation, damage control with a temporary shunt should be considered [74]. Definitive reconstruction can be performed once the patient is stable at the second laparotomy. Saphenous vein, femoral vein, and PTFE graft may all be used as conduit. Again, the use of autologous tissue is strongly encouraged because of the risks of bowel content spillage and subsequent graft infection. Proximal SMA partial transections can be managed with primary repair in 40 % of cases [75]. Additionally, there have been scattered reports of these injuries being treated with covered stent grafts [76–80]. This is a viable option in the critically ill patient, but long-term patency data is not yet available. Furthermore, these techniques are best utilized in the proximal segment of the SMA and should be avoided distal to the middle colic vessel to prevent covering important side branch vessels and inducing further bowel ischemia. All hematomas near the SMA should be explored in penetrating trauma. In blunt trauma, with a stable hematoma and viable nonischemic bowel, it is recommended to not explore the site. Instead, postoperative SMA evaluation via color-flow Doppler imaging, CT angiography, or angiography is recommended.
1.4.4 Renal Artery and Renal Parenchymal Injuries
Renal artery injuries are rare and account for about 0.05 % of all blunt trauma admissions [81]. The injuries typically present are intimal flaps, partial transections, pseudoaneurysms, traumatic AV fistulas, and acute occlusions. Treatment of traumatic renovascular injuries depends on the warm ischemia time, general condition of the patient, mechanism of injury, and condition of the contralateral kidney. Endovascular treatment should be considered the first-line therapeutic option for patients in stable condition with intimal tears, acute occlusions, false aneurysms, and arteriovenous fistulae. Because of the relatively large diameter, straight anatomical course, and parallel proximal and distal landing zones, endovascular interventions have shown great success in the treatment of renovascular injuries [82–84].
Expanding perinephric hematomas secondary to penetrating trauma should be explored, and most of these are found at the time of laparotomy for other associated injuries. A stable perinephric hematoma, away from the hilum may be considered an exception to this rule [85]. Blunt renovascular injuries are often found after a significant time delay. After 3–6 h of warm ischemia time, renal function is severely impaired. At this point, revascularization is of little use. In this circumstance, roughly 30–40 % of patients will ultimately develop renovascular hypertension [86–88].
1.4.5 Inferior Mesenteric Artery (IMA) Injuries
IMA injuries are rare and most often related to penetrating trauma. They account for less than 1 % of all abdominal vascular traumas [64]. Ligation is generally well tolerated and resultant colorectal ischemia is rare.
1.4.6 Iliac Artery Injuries
Isolated traumatic iliac vessel injuries carry a significant morbidity and mortality and are most often the result of penetrating trauma. In hospitals, mortality has been reported anywhere from 25 to 50 % depending on other associated injuries, the most significant being associated iliac vein injury [89–91]. In addition, iliac arterial injury has been reported numerous times after misadventures in lumbar spine surgery. Finally, with the increasing need for large-diameter transfemoral access for TEVAR and transfemoral aortic valve implantation (TAVI) cases, iatrogenic external iliac artery injury has increased.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


