Overview of Congenital Heart Disease
The excellent results obtained in infants and children using transthoracic echocardiography limit the need to use the transesophageal approach to elucidate lesions of congenital heart disease. In some patients, however, it is not possible to perform adequate transthoracic studies, particularly in adults with congenital heart disease. Furthermore, transesophageal echocardiography is the only practical method for assessing operative results during the procedure.
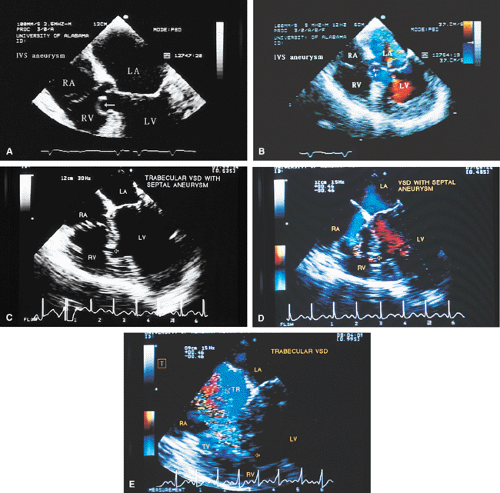
The septum primum covers the septum secundum and closes the fossa ovalis. In about 25% of patients this coverage is not complete, and a flow may occur across the septum. This is particularly likely in the setting of elevated right-sided pressures, which can open the patent foramen and result in paradoxic embolus. The secundum defect occurs at the level of the fossa ovalis and is the commonest of atrial septal defects. These lesions often present as a single large defect but may be fenestrated or multiple. Defects <2 cm in diameter can be closed by transcatheter patch closure, but larger defects must be closed surgically. The size of the defect is easily assessed by transesophageal echocardiography, especially if the multiplane probe is used.
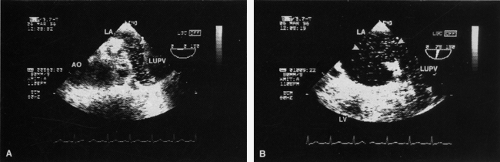
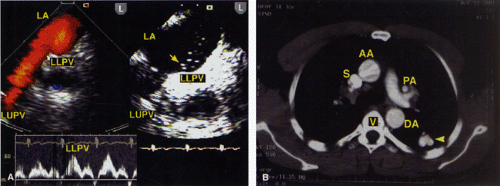
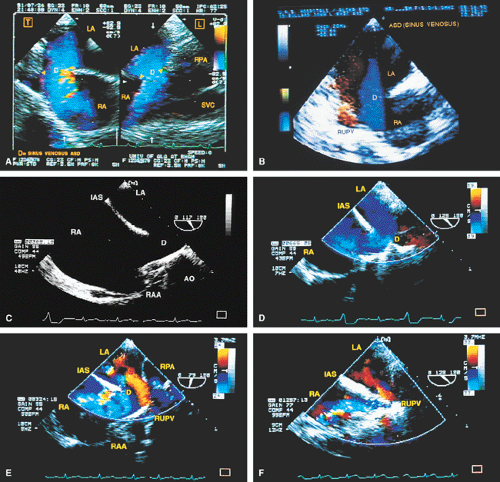
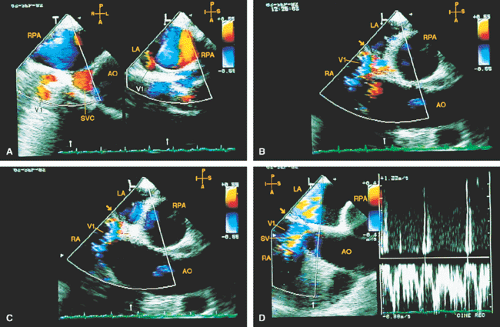
The ostium primum type of atrial septal defect (partial atrioventricular septal defect or partial atrioventricular canal defect) occurs at the base of the septum. It is often associated with a cleft in the anterior mitral valve leaflet that makes it regurgitant. In contrast, sinus venosus atrial septal defects occur in the superior part of the septum. Drainage of the right upper pulmonary vein into the right atrium is a commonly associated anomaly. The superior vena cava may override the defect. In adults, this defect may be difficult to visualize by transthoracic echocardiography. When an atrial septal defect is suspected on the basis of other findings but is not found on transthoracic examination, transesophageal echocardiography is indicated.
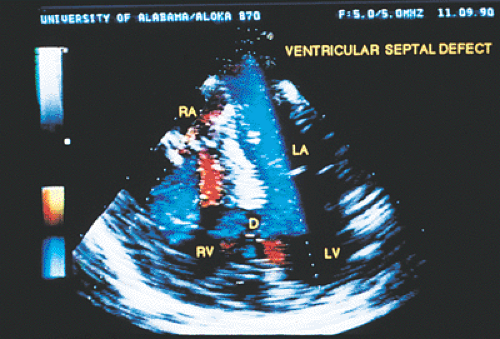
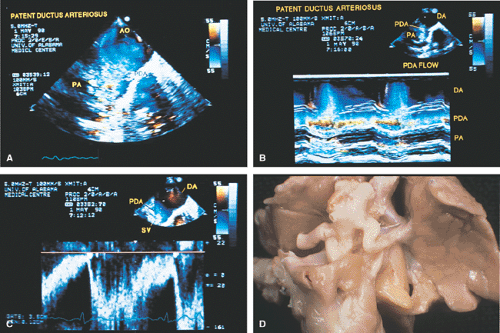
Ventricular septal defects, especially small ones, are often visualized more effectively on transthoracic echocardiography than on transesophageal echocardiography because more planes are available from the transthoracic approach. Patent ductus arteriosus and other aortopulmonary communications, both congenital and surgically created, can be visualized by transesophageal echocardiography.
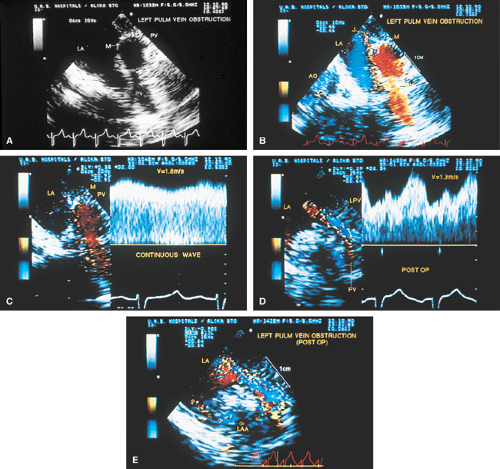
Stenosis of the pulmonary veins is usually diagnosed early. It can be seen as a narrowing of the vein, and Doppler examination demonstrates a high-velocity narrow jet with turbulence and spectral broadening. In adults, congenital stenosis must be differentiated from acquired narrowing produced by mediastinal lesions such as a tumor or sclerosing mediastinitis and pulmonary vein obstruction occurring after lung transplantation. In cor triatriatum, a membrane partitions the left atrium (or, rarely, the right atrium) into two chambers in such a way that the pulmonary veins are on one side of the membrane and the left atrial appendage is on the other side. Cor triatriatum is different from a supravalvar mitral membrane, which is located below and inferior to the left atrial appendage. These lesions may or may not result in obstruction to the flow. Congenital mitral stenosis is a rare lesion, similar in presentation to mitral stenosis. The valve is thickened and domes in diastole. Associated abnormalities of the papillary muscle and chordae may be present.
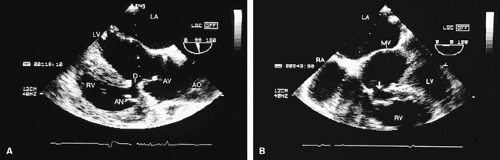
Subvalvular aortic stenosis results from a fibromuscular membrane that has a location that is variable relative to the aortic valve. These membranes are most commonly attached to the base of the anterior mitral leaflet and to the ventricular septum. Subvalvular membranes appear as linear echoes in the left ventricular outflow tract. Turbulent flow and a high gradient may be present, and there is often associated aortic regurgitation. Early, as opposed to
mid- or late, systolic closure of the aortic valve is commonly seen. It may involve only one cusp.
mid- or late, systolic closure of the aortic valve is commonly seen. It may involve only one cusp.
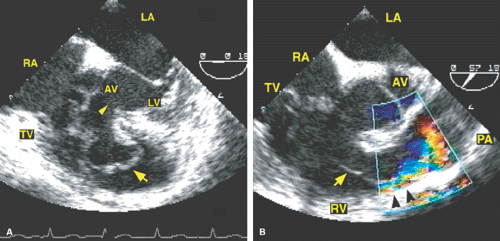
In congenital aortic stenosis the valve domes in systole. The short-axis view shows a typical “circle within a circle” appearance. Careful planimetry yields a good estimate of the valve area. Care must be taken to move the probe up and down the esophagus to visualize even the smallest flow-limiting orifice at the top of the domed valve. Color Doppler allows visualization of the width of the jet. A jet that is 7 mm or smaller suggests severe aortic stenosis in an adult. The jet width should be measured at its origin from the aortic valve. Color Doppler should be turned off during planimetry of the orifice because it tends to overestimate the area. It is, however, useful in identifying the aortic orifice in patients with heavily calcified valves that show no discernible opening movement in systole (i.e., fixed orifice). In these cases, the first appearance of turbulent flow signals in early systole helps identify the stenotic orifice, which can then be studied through planimetry with the color Doppler turned off. After valvotomy the jet size is larger, and the planimetered area is also greater. Because the transesophageal study can be performed intraoperatively, it is useful in guiding surgery. Supravalvular aortic stenosis is a rare lesion that may present as a discrete membrane or may be tubular. Coarctation of the aorta is best seen on transthoracic echocardiography but may also be evaluated by the transesophageal approach. The best results are obtained with a multiplane probe.
Membranes occur in the right atrium. Examples include eustachian valves and Chiari networks. Obstruction is rare but may occur. Right ventricular muscle bundles may be prominent and are associated with right ventricular hypertrophy. Rarely, the hypertrophy may be sufficient to cause obstruction in the body of the ventricle. A small ventricular septal defect may be associated, and it is suspected that the jet from the defect may, at least in some cases, stimulate hypertrophy. Infundibular stenosis may occur alone or in association with tetralogy of Fallot. The right ventricular outflow tract is best viewed in the long axis using longitudinal plane examination.
In congenital pulmonary stenosis the valve domes in systole and may also be thickened. Stenosis in the proximal segments of the right or left pulmonary arteries can be visualized by transesophageal echocardiography; however, stenosis in the distal segments or in the peripheral branches cannot be visualized using echocardiographic techniques.
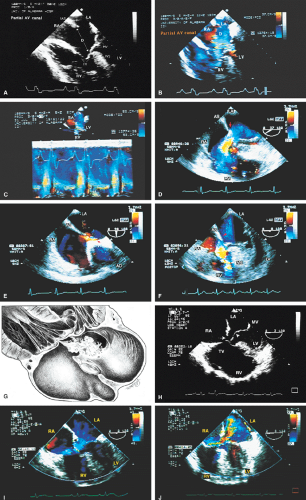
The pathophysiologic features of complete atrioventricular canal defect (atrioventricular septal defect), including absence of the atrioventricular septum, deficiency of the adjacent atrial and inlet ventricular septum, a common atrioventricular annulus, and shunting from the left atrium into the right atrium and from the left ventricle into the right ventricle, are well seen using the transesophageal approach. The severity of atrioventricular valve regurgitation can also be evaluated.
In tricuspid atresia, the tricuspid valve is absent and both atrial and ventricular septal defects are necessarily present to ensure patient survival. This lesion is often associated with a hypoplastic right ventricle. For the purpose of surgical planning, it is necessary to delineate the right ventricular outflow tract, the pulmonary valve, and the pulmonary artery. Surgical correction (i.e., the Fontan procedure) involves closure of the atrial septal defect, closure of any ventricular septal defect, and placement of a shunt from the right atrium to the pulmonary artery.
In transposition of the great vessels, the connection of the vessels to the ventricles can be seen and the relation of the pulmonary artery and the aorta can be delineated. Associated pulmonary valve stenosis can be seen, as can banding of the pulmonary artery. Coexisting atrial or ventricular septal defects and atrioventricular or semilunar valve regurgitation can also be assessed by transesophageal echocardiography. The adequacy of the Mustard procedure can be assessed immediately after the operation. Intra-atrial baffle leak or obstruction can be delineated. In congenitally corrected transposition of the great vessels, the discordant atrioventricular and ventriculoarterial connections, together with the presence and degree of Ebsteinization of the left-sided atrioventricular valve, can be assessed by transesophageal echocardiography.
Ebstein anomaly is characterized by the apparent displacement of one or more tricuspid valve leaflets into the right ventricle, resulting from a variable extent and degree of plastering of the leaflets to the contiguous ventricular septum or right ventricular wall. The degree of this displacement determines the size of the functional right ventricle. Transesophageal echocardiography is useful in assessing both these abnormalities and the severity of tricuspid regurgitation, which is often considerable. A patent foramen ovale or an atrial septal defect may be present, and may result in right-to-left shunting and cyanosis because of increased right atrial pressure. The anatomic features and the flow patterns of other complicated lesions, such as tetralogy of Fallot, double outlet right ventricle, and single ventricle can also be assessed through the transesophageal approach.
Abnormalities of the coronary arteries can be seen well. These include aneurysms, thrombi, and anomalous origins and course of the coronary arteries.
An important lesion is the sinus of Valsalva aneurysm. It may appear as a tube-like structure protruding into the right ventricle or the right atrium. Rupture may result in shunting into the right ventricle or the right atrium.
In the first part of this chapter the most common shunt lesions are illustrated, followed by the obstructive lesions (both left-sided and right-sided), and, finally, the more complex congenital pathologies. The second and third parts of this chapter supplement the material shown in the first part and provide an enhanced understanding of the role of transesophageal echocardiography in the assessment of congenital cardiac lesions.
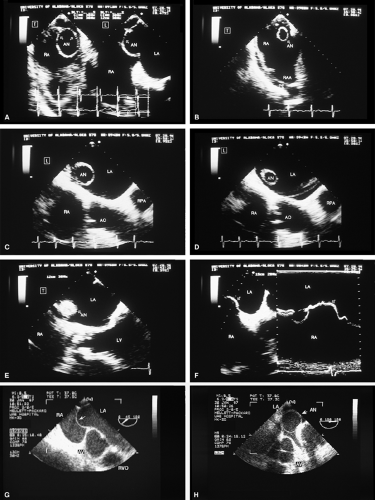
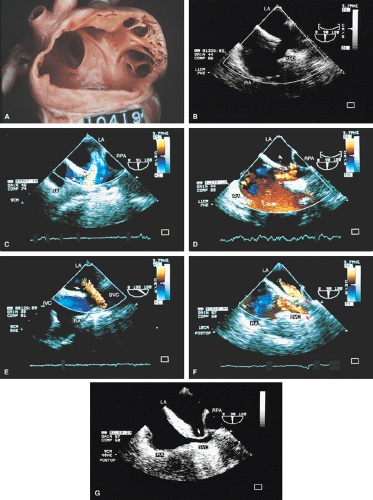
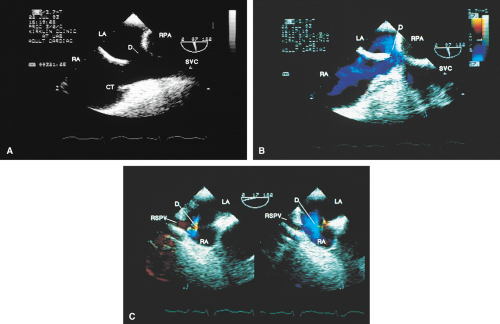
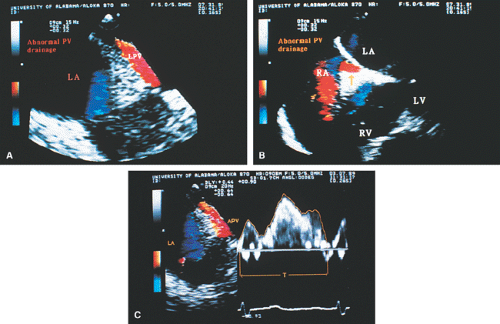
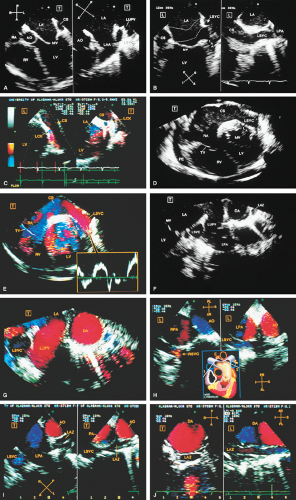
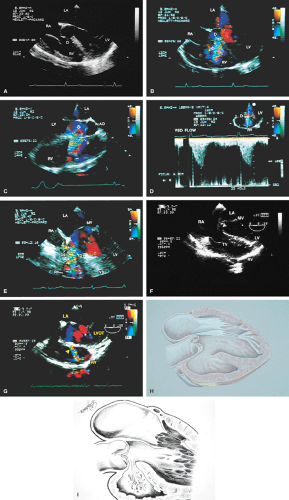
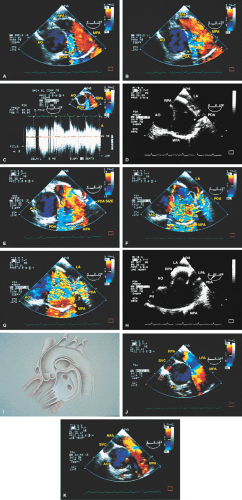
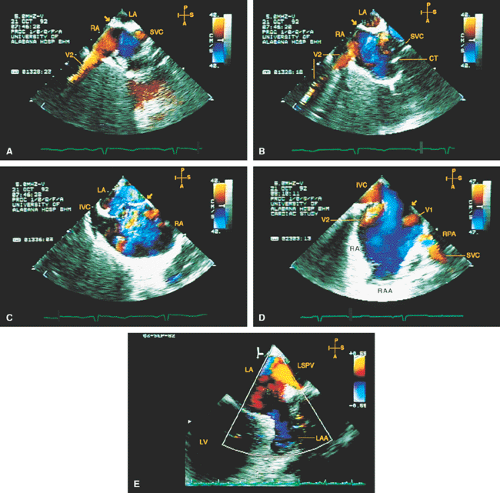 FIGURE 8.1.13. Isolated right-sided anomalous pulmonary venous return into the right atrium (RA). Same patient as in Figure 8.1.11. A–D. Longitudinal planes. A,B. The extracardiac course and the entrance of the second anomalous pulmonary vein (V2) into the RA are seen. C,D. The inferior vena cava (IVC) is separately visualized and enters the RA more posteriorly. E. The left superior pulmonary vein (LSPV) enters the left atrium (LA) normally. CT, crista terminalis; RAA, right atrial appendage; RPA, right pulmonary artery; SVC, superior vena cava. (Reproduced with permission from Sanyal RS, Nanda NC, Snell D, et al. Transesophageal echocardiographic findings of complete unilateral anomalous pulmonary venous connection of right lung to right atrium. Echocardiography 1994;11:93–100. ) |
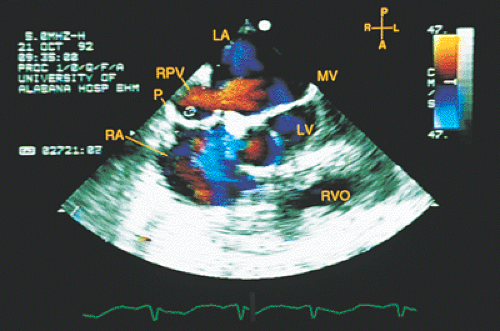 FIGURE 8.1.14. Isolated right-sided anomalous venous return into right atrium (RA). Same patient as in Figures 8.1.11 and 8.1.12. Transverse plane. Following surgery, pulmonary vein flow signals from the right side (RPV) are seen entering the left atrium (LA) posterior to the surgically inserted patch (P). LV, left ventricle; MV, mitral valve; RVO, right ventricular outflow tract. (Reproduced with permission from Sanyal RS, Nanda NC, Snell D, et al. Transesophageal echocardiographic findings of complete unilateral anomalous pulmonary venous connection of right lung to right atrium. Echocardiography 1994;11:93–100. ) |
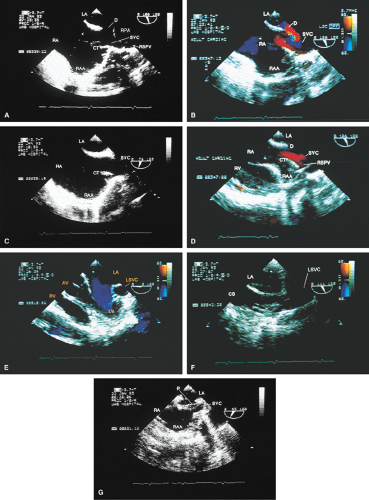
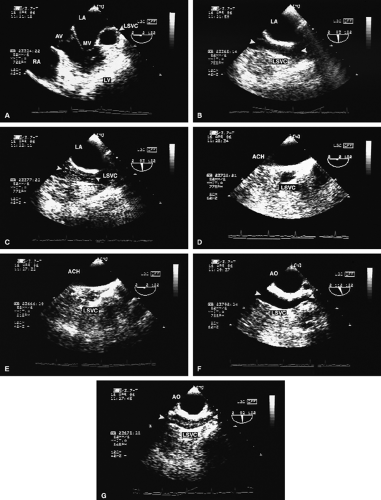
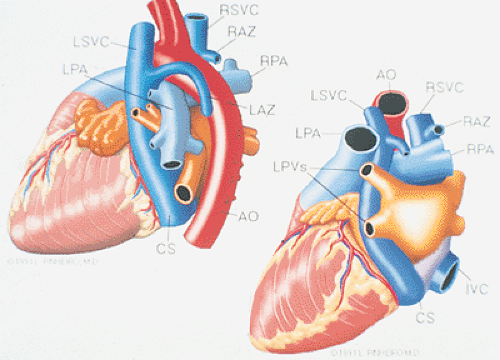
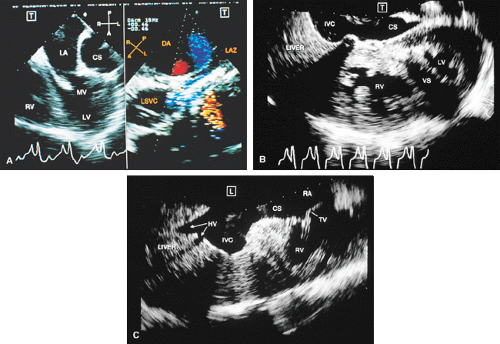
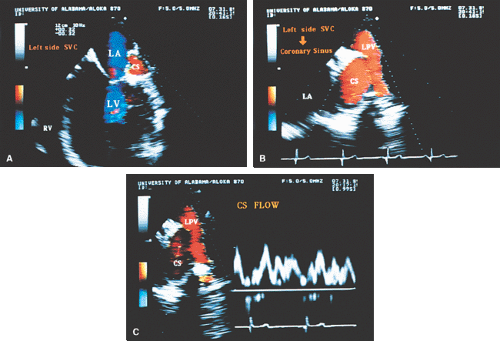
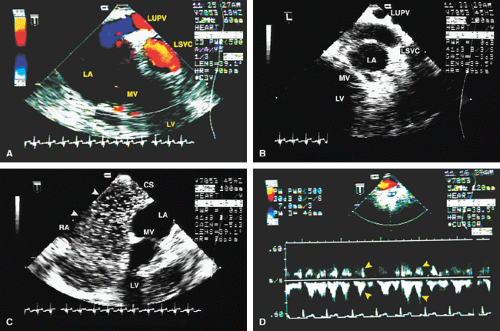
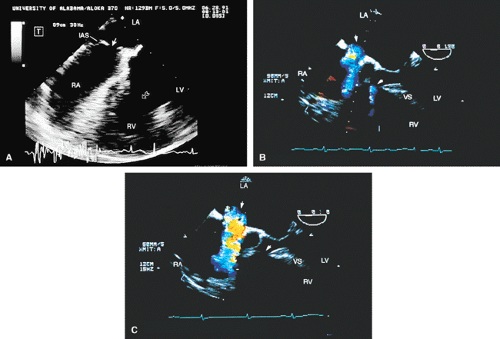
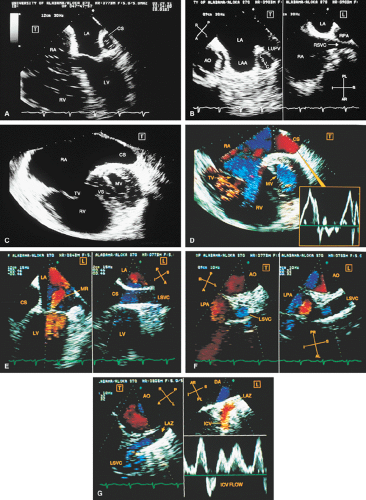 FIGURE 8.1.18. Left-sided superior vena cava (LSVC) draining into the coronary sinus. A 40-year-old woman, status postrepair of an atrial septal defect, presented with systemic hypertension, left ventricular hypertrophy, and mitral regurgitation (MR). A. Four-chamber view shows a dilated coronary sinus (CS) resulting from the drainage of the LSVC, noted at surgery for repair of atrial septal defect. Contrast injection into a left arm vein using normal saline demonstrates contrast echoes in the CS, right atrium (RA), and right ventricle (RV). B. Transverse plane examination (left) at the level of the aortic root (AO) demonstrates the relationship of the LSVC to the left atrial appendage (LAA) and left upper pulmonary vein (LUPV). Longitudinal plane examination (right) demonstrates the coexistence of a right-sided superior vena cava (RSVC). C,D. Composite illustrations prepared by combining two transverse plane images obtained by rotation of the transesophageal probe demonstrate an enlarged CS entering the RA. The inset in D represents a spectral trace obtained by color Doppler—guided pulsed Doppler interrogation of the CS. E. Longitudinal plane examination. The two-chamber view (left) shows marked enlargement of the CS. Note also the presence of MR in this systolic frame. Withdrawing the probe and rotating it counterclockwise (to shift the plane to the left) brings into view the entrance of the LSVC into the CS (right). F. The probe is withdrawn further to the level where the distal ascending aorta (DA) and the main pulmonary artery bifurcation are visualized. When the probe is rotated counterclockwise (to move the plane to the left), it demonstrates the LSVC (imaged in short axis) to be located posterior to the left pulmonary artery (LPA) and in the vicinity of the aorta (AO) in this transverse plane examination (left), in contrast to Figure 8.1.11, where the LSVC was located anterior to the LPA. In the longitudinal plane examination (right), the LSVC is imaged in long axis. G. Withdrawing the probe and rotating it counterclockwise to image the proximal DA brings into view the LAZ, which is seen to enter LSVC in the transverse plane examination (left). Longitudinal plane examination (right, top) shows a posterior intercostal vein (ICV) joining the LAZ posterior to the DA, viewed in long axis. The spectral trace (right, bottom) was obtained by color Doppler—guided pulsed Doppler interrogation of the ICV. LA, left atrium; LAZ, left azygos vein; LV, left ventricle; MV, mitral valve; RPA, right pulmonary artery; TV, tricuspid valve; VS, ventricular septum. (Reproduced with permission from Nanda NC, Pinheiro L, Sanyal R, et al. Transesophageal echocardiographic examination of left-sided superior vena cava and azygos and hemiazygos veins. Echocardiography 1991;8:731–740. ) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree