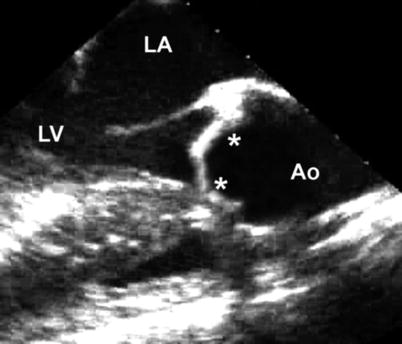
Fig. 11.1
(a) Deep transgastric long axis view at 0° showing the LV outflow tract; (b) Deep transgastric sagittal view at 90° showing the RV outflow tract; (c) Illustration depicting how the LV and RV outflow tracts are evaluated in deep transgastric views (Ao aorta, LV left ventricle, MPA main pulmonary artery, RV right ventricle)
Occasionally, TEE is necessary outside of the operating room for older patients with poor transthoracic echocardiographic windows, particularly if the patient has undergone prior surgery.
Normal Anatomy
The term conotruncus (from the Greek words conus for cone and truncus for trunk or body) represents the outflow tracts of both ventricles. It is composed of the subarterial regions, the semilunar valves, the great arterial roots, and the proximal trunks. The conus (also known as the infundibulum from the Latin word for funnel) defines the subarterial muscular chamber, which prevents fibrous continuity between the semilunar valve and the corresponding atrioventricular valve. In the normal heart, the subpulmonary conus prevents fibrous continuity between the tricuspid valve and the PV. The supraventricular crest is the prominent muscular shelf along the posterior aspect of the subpulmonary conus, and it is composed of the conal septum (or infundibular septum) separating the RVOT from the LVOT, the right ventriculo-infundibular fold separating the tricuspid valve leaflets from the PV leaflets, and the subpulmonary muscular sleeve supporting the PV leaflets. The subpulmonary conus is separated from the trabecular segment of the RV chamber at the infundibular os, an area defined by the moderator band, the septal band (also known as the septomarginal trabeculation), and the parietal band of the RV. The subaortic conus regresses in utero, resulting in varying degrees of fibrous continuity between the mitral valve and AoV. This fibrous area, known as the mitral-aortic intervalvular fibrosa, is measured as the shortest distance from the anterior mitral leaflet to the base of the non-coronary or left coronary AoV leaflet and can be elongated in cases of subvalvar AS and tetralogy of Fallot.
The normal semilunar valve is a three-dimensional structure wherein three leaflets (or cusps) are attached to the arterial root and supporting ventricular muscle in a semilunar or crown-like fashion. The leaflets are separated by three commissures that extend during diastole from the center of the valve at the level of the ventriculo-arterial junction to the periphery at the level of the sino-tubular junction (Fig. 11.2, Video 11.2). It is important to recognize that the semilunar valve “annulus” is in fact a diagnostician construct without a true anatomic correlate since the semilunar valve leaflet attachments extend from the anatomic ventriculo-arterial junction up to the sino-tubular junction; the so-called “annulus” as defined in echocardiography represents only the most proximal attachment of the semilunar valve and not its attachment in entirety [7] (Fig. 11.3, Video 11.3).
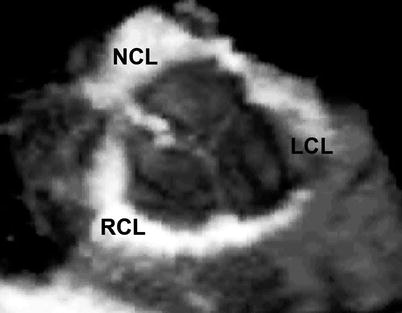
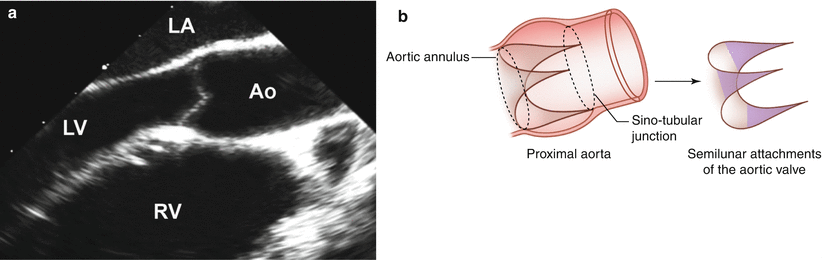

Fig. 11.2
Three-dimension mid esophageal aortic valve short axis view depicting the three-dimensional nature of a normal tri-leaflet aortic valve (LCL left coronary leaflet, NCL non-coronary leaflet, RCL right coronary leaflet)

Fig. 11.3
(a) Mid esophageal aortic valve long axis view at 120° showing the LV outflow tract; (b) Illustration of the aortic valve and its semilunar attachments within the aortic root from the ventriculo-arterial junction to the sino-tubular junction (Ao aorta, LA left atrium, LV left ventricle, RV right ventricle)
Left Ventricular Outflow Tract Anomalies
Valvar Aortic Stenosis
Congenital valvar AS results from a bicuspid or unicuspid AoV, a dysplastic tricuspid AoV, or a hypoplastic aortic “annulus”. Acquired valvar AS usually results from calcification secondary to degenerative or post-inflammatory causes [8], though a bicuspid AoV is also a risk factor for calcification [5]. A bicuspid AoV is the most common cause of valvar AS, although the presence of a bicuspid AoV does not necessarily mean a patient has (or will develop) valvar AS. As noted previously, a bicuspid AoV is the most common congenital heart defect, with an incidence of 0.4–2 % in the general population [3–5]. There are several distinct anatomic variations. It is rare to encounter a bicuspid AoV with only two well-developed leaflets (Fig. 11.4, Video 11.4); instead, it usually involves fusion of two of the three leaflets or underdevelopment of one of the commissures (known as a raphé) [9]. It is because of the absence of one of the three commissures that the lesion is often referred to as a bicommissural AoV. Among these, fusion occurs most commonly at the intercoronary commissure between the right and left coronary leaflets with a frequency of 70 % (Fig. 11.5, Video 11.5), followed by the commissure between the right and non-coronary leaflets with a frequency of 28 % (Fig. 11.6, Video 11.6); fusion at the commissure between the left and non-coronary leaflets is very rare [10]. In adults, the rate of AS progression appears to be higher in those valves with fusion of the intercoronary commissure [11], though studies in children have revealed more rapid progression of AS and regurgitation as well as shorter time from diagnosis to intervention in valves with fusion of the commissure between the right and non-coronary leaflets [12]. Associations with a bicuspid AoV include aortic coarctation [3] or interrupted aortic arch, subvalvar AS, a ventricular septal defect (VSD), a coronary anomaly, Turner syndrome [13], and aortic dilation or aneurysm formation [14–16]. Valvar AS can be an evolving pathology and the degree of progression may be associated with age at presentation and/or severity at the time of presentation [17]. Although it occurs rarely, sudden death in this patient population appears to be related to significant stenosis and regurgitation [18]. In addition, the chronic pressure load experienced by the LV leads to hypertrophy with the potential for permanent muscular damage and fibrosis. Therefore, management of these patients must focus on prevention of these sequelae.
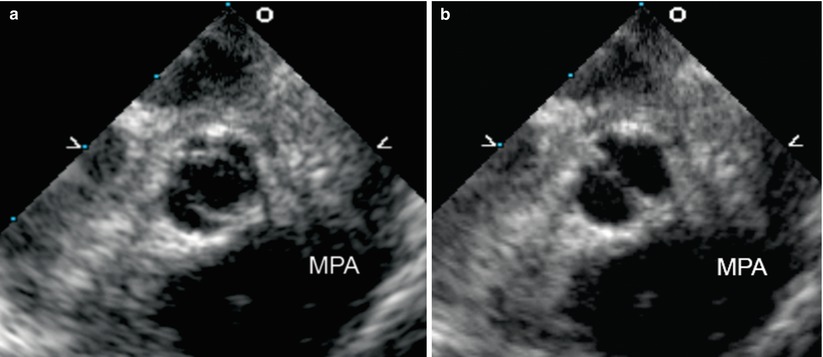
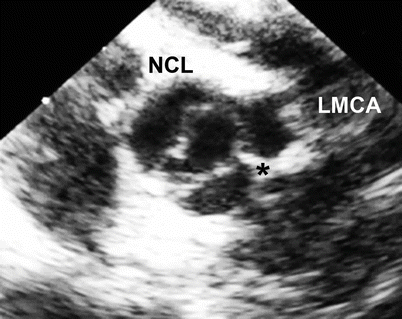
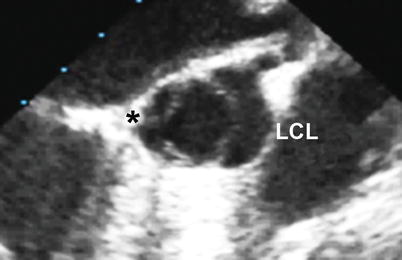

Fig. 11.4
Mid esophageal aortic valve short axis view at 30° shows a true bicuspid aortic valve with only two leaflets (the right and left coronary leaflets) and absent non-coronary leaflet, in the open (a) and closed (b) positions. This gives a “fishmouth” appearance to the valve orifice (MPA main pulmonary artery)

Fig. 11.5
Mid esophageal aortic valve short axis view at 30° showing a bicuspid aortic valve with fusion or underdevelopment of the intercoronary commissure (black asterisk) (NCL non-coronary leaflet, LMCA left main coronary artery)

Fig. 11.6
Mid esophageal aortic valve short axis view at 30° showing a bicuspid aortic valve with fusion or underdevelopment of the commissure between the right and non-coronary leaflets (black asterisk) (LCL left coronary leaflet)
Over the past several decades, transcatheter balloon valvotomy in the catheterization laboratory has become a more popular management approach for patients with valvar AS, superseding surgical valvotomy as the primary mode of treatment. TEE is not often needed during this procedure, though occasionally it is helpful in determining the appropriate intervention and/or assessing its efficacy. Echocardiography for valvar AS should always include assessment of leaflet and commissural morphology, degree of obstruction, degree of LV hypertrophy, ascending aortic size, and left atrial dilation. Aortic measurements (aortic valve, aortic root, and ascending aorta) are often helpful. Leaflet and commissural morphology are best evaluated in the mid esophageal views, usually at 30–45° for the mid esophageal aortic valve short axis (ME AV SAX) images (Figs. 11.4, 11.5, 11.6, and 11.7, Videos 11.4, 11.5, 11.6, and 11.7) and at ~120° for the mid esophageal aortic valve long axis (ME AV LAX) images (Fig. 11.8, Video 11.8). It is, however, important to recognize that pure short and long axis images can be difficult to obtain—even with multiplane TEE—because of the fixed spatial relationship between the esophagus and the aortic outflow tract. When a pure short axis image is available (that is, when the AoV annular plane is completely perpendicular to the axis of the beam), the relative sizes of the leaflets should be evaluated, and failure of commissural opening in the setting of a bicuspid or unicuspid valve can be displayed. Long axis imaging of the AoV can reveal thickened and doming leaflets. More recently, three-dimensional echocardiography has emerged as an important tool for the assessment of aortic valve anatomy and pathology (Chaps. 19 and 20).
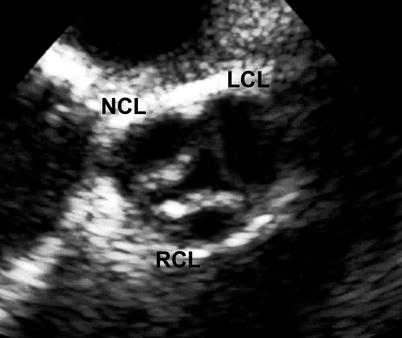
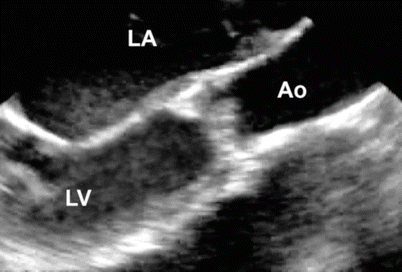

Fig. 11.7
Mid esophageal aortic valve short axis view at 30° showing a dysplastic aortic valve with thickened right and non-coronary leaflets (LCL left coronary leaflet, NCL non-coronary leaflet, RCL right coronary leaflet)

Fig. 11.8
Mid esophageal aortic valve long axis view at 120° showing a dysplastic aortic valve associated with valvar aortic stenosis (Ao aorta, LA left atrium, LV left ventricle)
The degree of obstruction is generally assessed by estimation of the pressure gradient across the stenotic valve, using continuous wave Doppler interrogation along the aortic outflow tract. This is best performed in the deep transgastric long axis (DTG LAX) view with multiplane angle between 0° and 30° (Fig. 11.1a, Video 11.1a), or sometimes from the deep transgastric sagittal (DTG Sagittal) view with multiplane angle between 80° and 110° (Fig. 11.1b, Video 11.1b). Because of the distance from the probe to the outflow tract, imaging from this view often requires using the lowest possible frequency for improved penetration as well as minimizing the image and color sector size. Every effort should be made to align the Doppler beam with the LVOT. As an alternative, the transgastric long axis view (TG LAX) can be used for interrogation of the LVOT because it generally provides favorable angles for continuous wave Doppler interrogation (Chap. 4) with a shorter distance from the probe to the aortic valve. Both peak and mean pressure gradients should be measured [19–21]. It is important to recognize that echocardiographically derived Doppler gradients often do not correspond to the peak-to-peak gradient measured in the catheterization laboratory, a common discrepancy which results from several factors, including a phenomenon called pressure recovery [22]. In addition, severe LV dysfunction in the setting of valvar AS is associated with low cardiac output and diminished transvalvar flow, and Doppler interrogation may reveal a low gradient despite severe obstruction at the valvar level. Similarly, the degree of LVOT obstruction can be artificially decreased in the setting of general anesthesia, deep sedation, or hypovolemia. In addition to pressure gradient, some use calculated aortic valve area to evaluate the degree of obstruction as derived by the continuity equation, which states that the stroke volume in the subaortic region is the same as the stroke volume at the valvar level (see Chap. 1). Since stroke volume is equal to the velocity-time integral multiplied by cross-sectional area at a particular location, the effective aortic valve area can be calculated if the LVOT cross-sectional area and the velocity-time integrals at the LVOT and at the aortic valve are known [23]. However, the LVOT is frequently not circular in shape, precluding accurate assessment of LVOT cross-sectional areas using LVOT diameters, and some have suggested that three- dimensional TEE may be preferable to two-dimensional imaging for more accurate assessments of aortic valve area [24].
It is important to note that AS represents a disease continuum, and severity cannot be defined by a single value [21]. With these considerations in mind, the following echocardiographic criteria for classification of AoV stenosis severity were recently given by a 2008 American College of Cardiology/American Heart Association (ACC/AHA) task force on valvular heart disease [21].
Mild: peak velocity <3 m/s, mean gradient <25 mmHg, valve area >1.5 cm2
Moderate: peak velocity 3.0–4.0 m/s, mean gradient 25–40 mmHg, valve area 1.0–1.5 cm2
Severe: peak velocity >4 m/s, mean gradient >40 mmHg, valve area <1.0 cm2
Similar guidelines have not been written specifically for the pediatric population. At present, the above criteria are generally applied to this group of patients as well [25–27].
LV hypertrophy is best evaluated in the transgastric basal short axis (TG Basal SAX) and transgastric mid short axis (TG Mid SAX) views at 0°, though a quantitative assessment of the degree of hypertrophy may not be reliable with TEE, again because pure short axis images of the LV in transgastric views at 0° may not always be available. The size of the aortic root and ascending aorta are best measured in mid esophageal aortic valve long axis (ME AV LAX) and mid esophageal ascending aortic long axis (ME Asc Ao LAX) views at 90–120°; important measurements that should be obtained in early to mid systole include the diameters of the aortic valve annulus, aortic root diameter, sino-tubular junction, and ascending aorta [28]. These measurements are analogous to those obtained by transthoracic echocardiography, and they can be compared to published normal values [29, 30]. Left atrial dilation can be easily visualized in mid esophageal fourchamber (ME 4 Ch) and twochamber (ME 2 Ch) views at 0° and 90° respectively.
Recently, a new procedure for the nonsurgical treatment of severe valvar AS has garnered widespread interest. Known as transcatheter AoV replacement/implantation, or TAVR/TAVI, this procedure is performed in the cardiac catheterization laboratory and involves the implantation of a bovine or porcine pericardial valve mounted upon a metal support frame [31]. Delivered transarterially (access by percutaneous femoral or subclavian artery, or through the left ventricular apex), the balloon-mounted valve is advanced to the AoV position, and the balloon expanded within the diseased AoV, displacing the diseased native valve leaflets. During the procedure, TEE is utilized and serves a number of important functions: (a) pre-procedure evaluation of anatomy and function; (b) monitoring of valve implantation; (c) post-implantation evaluation of valve function and valve regurgitation (Fig. 11.9, Video 11.9). The post-implantation TEE is particularly useful in that it enables accurate differentiation between transvalvar and paravalvar aortic regurgitation, something not easily visualized by angiography or fluoroscopy [32]. At present, two major valves are available—the Edwards SAPIEN XT heart valve (Edwards Lifesciences Inc., Irvine, California) and the CoreValve ReValving system (Medtronic Inc., Minneapolis, Minnesota)—but a number of other valves are undergoing early clinical evaluation [33]. Currently, the procedure is performed only in adults; candidates are generally patients with severe valvar AS felt to be too high risk for surgical intervention [34, 35] (see also Chap. 16).
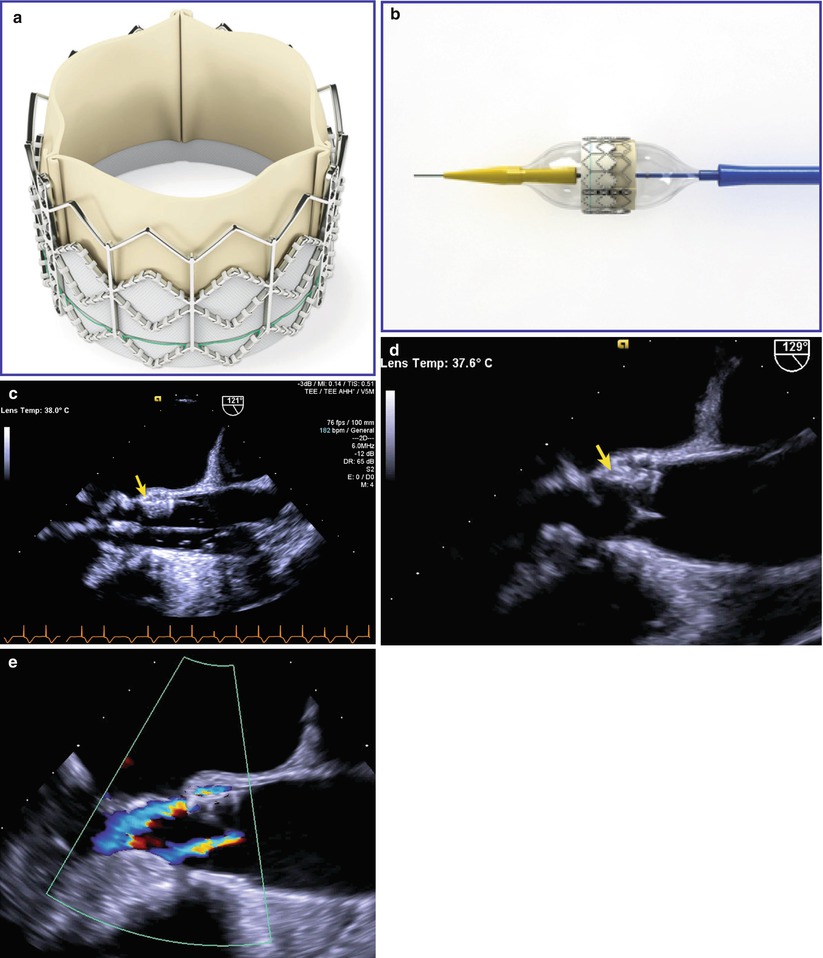

Fig. 11.9
Transcatheter aortic valve replacement/implantation (TAVR/TAVI). (a) Shows the Edwards SAPIEN valve (Edwards Lifesciences Inc., Irvine, California), a bovine pericardial valve mounted on a stainless steel frame. (b) Shows the same valve mounted on a balloon catheter that is used for valve delivery. (c–e) Are transesophageal echocardiographic images obtained from the mid esophageal aortic valve long axis view at 120–130°. (c) Shows the balloon being inflated (device indicated with arrow), and (d) Shows the valve (arrow) after implantation. In (e), following valve implantation color flow Doppler shows two small jets of regurgitation: one a central transvalvar jet, the other a peripheral paravalvar jet (Echocardiographic images provided courtesy of Siemens Medical Systems USA, Inc. © 2012–13 Siemens Medical Solutions USA, Inc. All rights reserved)
Subvalvar Aortic Stenosis
Subvalvar AS is rarely diagnosed in utero or in the newborn period, prompting many people to classify this lesion as an acquired heart disease rather than a congenital one [36, 37]. It is a progressive disease, particularly in children, and some have suggested that its development may involve the following mechanisms: abnormal LVOT morphology leads to increased septal shear stress, which because of some genetic predisposition leads in turn to cellular proliferation [38, 39]. Some of the abnormalities in LVOT morphology that are associated with subvalvar AS include a steep aorto-septal angle (Fig. 11.10a, Video 11.10), an elongated mitral-aortic intervalvular fibrosa, exaggerated aortic override [40, 41], prominent LVOT muscle bundles, and abnormal mitral valve attachments to the ventricular septum. In addition, the distance from the level of the obstruction to the AoV appears to be one of the major predictors of significantly progressive obstruction [42] (Fig. 11.10b, Video 11.10). Subvalvar AS can occur without or with a VSD. Examples of subvalvar AS in the absence of a VSD include: (a) a discrete fibrous or fibromuscular subaortic ridge protruding from the ventricular septum and sometimes extending to the anterior mitral leaflet (Fig. 11.10b, Video 11.10); (b) an extensive muscular shelf creating a tunnel-like LVOT; (c) hypertrophic cardiomyopathy with dynamic obstruction secondary to diffuse septal hypertrophy and systolic anterior motion of the mitral valve and/or its tensor apparatus (Fig. 11.11, Video 11.11). Subvalvar AS without a VSD can also develop postoperatively after mitral valve replacement with a mechanical prosthesis [43–45]. The most common type of subvalvar AS in the setting of a VSD occurs with posterior deviation of the conal septum, a lesion that is often associated with aortic coarctation or interrupted aortic arch [46, 47]. (see Chaps. 9 and 13) Other examples of subvalvar AS include endocardial folds or fibromuscular ridges at the crest of the muscular septum (Fig. 11.12, Video 11.12), often without significant obstruction [48], and abnormal mitral valve attachments to the ventricular septum, often in the setting of an atrioventricular canal (atrioventricular septal) defect (see Chap. 8) Aside from a VSD, subvalvar AS can also be associated with a bicuspid AoV, a double-chambered RV (DCRV) [49], and aortic regurgitation (AR). In fact, the risk for rapidly progressive AR is frequently the reason that children with subvalvar AS require early surgical intervention [50], although AR in adults with subvalvar AS is rarely progressive [51, 52].
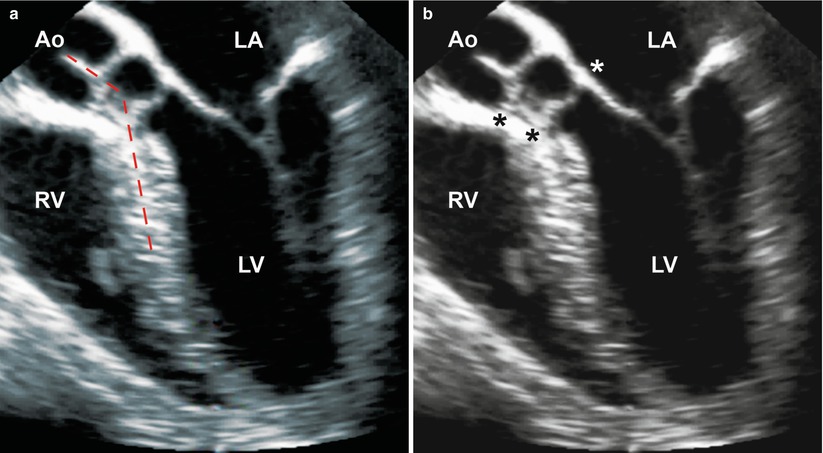
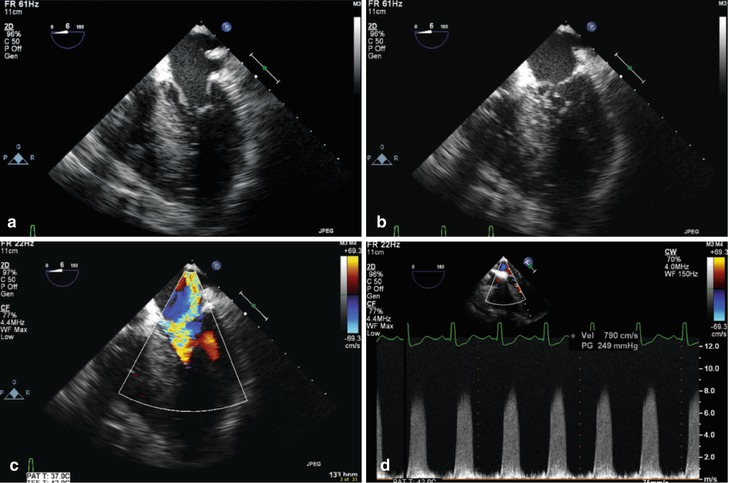
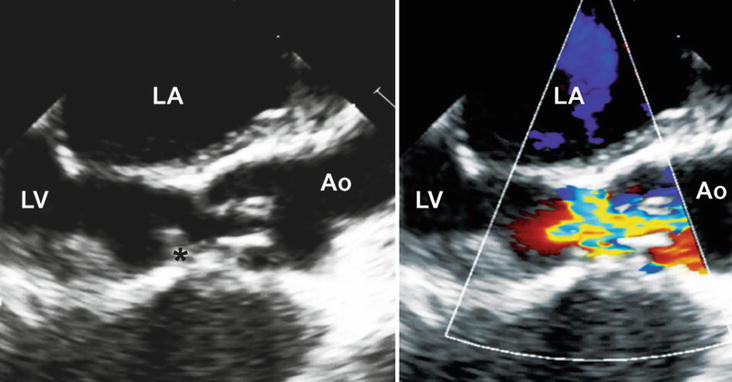

Fig. 11.10
Mid esophageal four chamber view with probe anteflexion to visualize the left ventricular outflow tract, showing a subaortic fibromuscular ridge associated with subvalvar aortic stenosis. The two figures depict (a) a steep aorto-septal angle (dashed red lines), and (b) the distance between the ridge and the aortic annulus (black asterisk) and extension of the fibromuscular ridge to the anterior mitral leaflet (white asterisk) (Ao aorta, LA left atrium, LV left ventricle, RV right ventricle)

Fig. 11.11
Mid esophageal four chamber view showing subaortic stenosis causing systolic anterior motion (SAM) of the mitral valve, in a patient with significant ventricular septal hypertrophy. This is characterized by systolic displacement of the anterior mitral leaflet into the left ventricular outflow tract. In diastole (a) the mitral valve opens normally. However during systole there is a “drag” effect produced by the underfilled, hyperdynamic left ventricle that pulls the anterior leaflet into the left ventricular outflow tract (b). This prevents the mitral valve from effective coaptation and produces significant mitral regurgitation (c). The SAM also results in dynamic left ventricular outflow tract obstruction, producing a gradient that can be very high; in this patient there is a late-peaking, dagger-shaped spectral Doppler tracing, with a peak velocity measured at nearly 8 m/s, or 256 mmHg (d)

Fig. 11.12
Mid esophageal aortic valve long axis view at 120° showing a subaortic fibromuscular ridge (black asterisk) and a dysplastic aortic valve resulting in combined subvalvar and valvar aortic stenosis in addition to a small membranous ventricular septal defect (not well-seen on the image, but readily visible on Video 11–12). The fibromuscular ridge is located at the crest of the muscular septum. Ao aorta, LA left atrium, LV left ventricle
TEE is especially useful in delineating the mechanism of obstruction in patients with subvalvar AS. A fibromuscular ridge with or without extension to the anterior mitral leaflet, posterior deviation of the conal septum, a tunnel-like LVOT, and abnormal mitral valve attachments to the ventricular septum can usually be characterized in the ME 4 Ch and ME AV LAX (at ~120°) views or in the DTG LAX view at 0–30° and DTG Sagittal view at 80–110°. In addition, the distance from a fibromuscular ridge to the AoV leaflets can be measured in these views (Fig. 11.10b). The degree of AR can usually be assessed in multiple mid esophageal and deep transgastric views (as discussed later in this chapter). The AoV leaflet and commissural morphology should always be evaluated in the ME AV SAX view at ~30° (Figs. 11.4, 11.5, 11.6, and 11.7), especially in the setting of significant AR. The utility and limitations of the DTG LAX view at 0–30° in quantifying the degree of obstruction have been discussed previously. Occasionally a ME Asc Ao LAX view at ~120°, or higher view such as the upper esophageal aortic arch long axis (UE Ao Arch LAX) at 0° or upper esophageal aortic arch short axis (UE Ao Arch SAX) at ~120° can provide an adequate Doppler angle to measure the gradient arising from the subaortic region. In some instances of subvalvar AS, a favorable angle for spectral Doppler interrogation can even be obtained from a modified ME 4 Ch view (Fig. 11.11, Video 11.11), though care must be taken not to contaminate the continuous wave Doppler tracing with a mitral regurgitation jet present along the same scan line. After mitral valve replacement with a mechanical prosthesis, the mid esophageal window can be ineffective because the mechanical prosthesis is located between the TEE probe and the LVOT, and acoustic interference by the prosthesis can partially or completely inhibit visualization of the outflow tract. In these instances, the TG LAX and deep transgastric views (DTG LAX, DTG Sagittal) can be helpful since the probe is positioned beyond the prosthesis, allowing for unobstructed imaging and Doppler interrogation. Associations such as a VSD or a DCRV should be excluded in the preoperative TEE, and these are usually visualized in the mid esophageal views: the ME 4 Ch view at 0° and the mid esophageal right ventricular inflow-outflow (ME RV In-Out) at 60–90°. Postoperatively, residual LVOT obstruction should be excluded qualitatively in the ME AV LAX and mid esophageal long axis (ME LAX) views (multiplane angle for both at ~120°), and quantitatively (by spectral Doppler evaluation) in the DTG LAX, DTG Sagittal, and TG LAX views. The AoV leaflets should be re-evaluated in the ME AV SAX view at 30–45°, and the presence of post-interventional AR should be assessed in multiple mid esophageal and deep transgastric views. Finally, inadvertent VSD creation and/or mitral valve injury are known complications following surgical intervention of subvalvar aortic stenosis [53, 54]. These areas should be evaluated carefully by postoperative TEE using a variety of mid esophageal and deep transgastric views, as described above and in Chap. 4.
Supravalvar Aortic Stenosis
Supravalvar AS is the least common type of LVOT obstruction, representing only 7 % of all patients undergoing surgical repair for LVOT obstruction at Children’s Hospital Boston from 1956 to 1976 [55]. Although it occasionally presents as a familial autosomal dominant lesion or as a sporadic idiopathic disorder, it is usually associated with Williams syndrome, a constellation of clinical features which include developmental delay, calcium metabolism problems, failure to thrive, and abnormal facial features [56]. Supravalvar AS results from a mutation or deletion of the elastin gene on chromosome 7 [57, 58]. The obstruction occurs at the sino-tubular junction, and three anatomic subtypes have been described: the hourglass type (most common) involving dilation of the aortic root proximal to the narrowing and the ascending aorta distal to the narrowing (Fig. 11.13, Video 11.13), the membranous or diaphragmatic type, and the rare tubular type with diffuse hypoplasia of the ascending aorta [59, 60]. Patients with Williams syndrome can also have branch pulmonary artery stenosis, aortic coarctation, and renal artery stenosis in addition to supravalvar AS. Other associations include subvalvar AS, an abnormal AoV, and coronary abnormalities such as coronary artery dilation, coronary thickening, ostial stenosis, and ostial entrapment by a tethered AoV (Fig. 11.13). Obstruction can occur at more than one level, and the degree of obstruction usually progresses over time. Surgical intervention is generally undertaken when significant obstruction is present in order to prevent irreversible left ventricular myocardial damage. In many cases, TEE is superior to transthoracic echocardiography in the evaluation of the entire extent of the ascending aorta. The aortic root and proximal ascending aorta are best assessed by TEE in a ME AV LAX view at ~120° wherein the long axis of the proximal aorta can be displayed (Fig. 11.13, Video 11.13). During probe withdrawal in this view, the ascending aorta can be further evaluated with the ME Asc Ao LAX view. The degree of obstruction can be quantified with DTG LAX (Fig. 11.14, Video 11.14), DTG Sagittal, and TG LAX views. In some cases, withdrawing the probe to an UE Ao Arch LAX or UE Ao Arch SAX view will provide an adequate Doppler angle of interrogation to quantify the degree of obstruction. TEE imaging, color mapping, and Doppler interrogation should also exclude other associations such as subvalvar AS (ME 4 Ch, ME AV LAX views), an abnormal AoV (ME AV SAX, ME AV LAX views), and coronary artery dilation and coronary ostial obstruction (ME AV SAX, ME AV LAX views). Evaluation of possible coronary ostial obstruction is best performed with color mapping looking for turbulence in the area of stenosis [61–63]. The TG Mid and Basal SAX views can also be used to evaluate for segmental wall motion abnormalities, supporting the possibility of coronary artery obstruction. The branch pulmonary arteries should also be evaluated for possible stenosis using the mid esophageal ascending aorta short axis (ME Asc Ao SAX), upper esophageal pulmonary artery LAX (UE PA LAX), and UE Ao Arch SAX views. The branch pulmonary arteries can be difficult to evaluate by TEE, though color mapping can be quite helpful in ascertaining the presence and location of the stenosis (Chaps. 4 and 13).
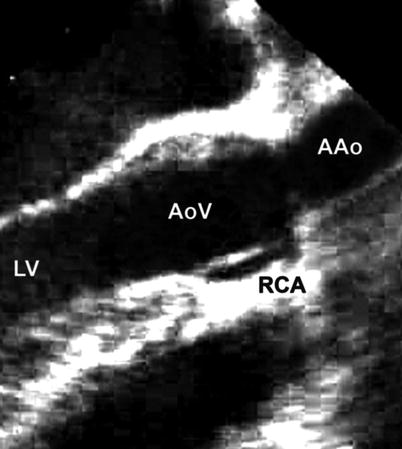
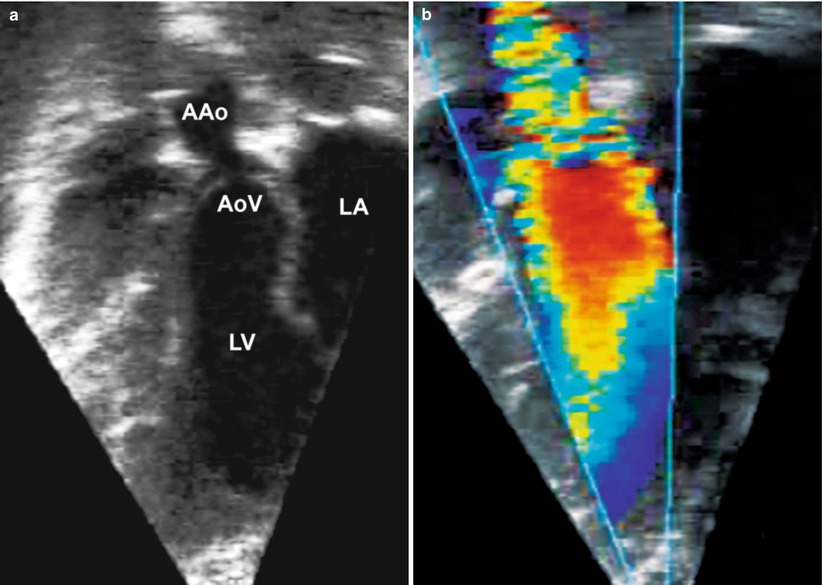

Fig. 11.13
Mid esophageal aortic valve long axis view at 120° showing discrete narrowing at the sino-tubular junction resulting in supravalvar aortic stenosis and entrapment of the right coronary ostium (AAo ascending aorta, AoV aortic valve, LV left ventricle, RCA right coronary artery)

Fig. 11.14
Deep transgastric long axis view at 30° showing discrete narrowing of the sino-tubular junction resulting in supravalvar aortic stenosis (a) two-dimensional imaging and (b) color flow mapping (AAo ascending aorta, AoV aortic valve, LA left atrium, LV left ventricle)
Aortic Regurgitation
Isolated congenital AR, defined loosely as diastolic flow from the aortic root into the LV, is a rare lesion [64]. Congenital etiologies causing AR in childhood include a bicuspid or quadricuspid AoV (Fig. 11.15, Video 11.15), a dysplastic tricuspid AoV, an aortico-left ventricular tunnel, absence of one or more AoV leaflets, a coronary-cameral fistula into the LV, and a ruptured sinus of Valsalva aneurysm into the LV. Occasionally AR occurs in the setting of subvalvar AS (as discussed previously), AoV prolapse into a membranous or doubly-committed subarterial VSD, or a ruptured sinus of Valsalva aneurysm into the right atrium (Fig. 11.16, Video 11.16a and 11.16b) or RV. It can also be seen with aortic root dilation in association with neonatal Marfan syndrome, tetralogy of Fallot, or truncus arteriosus. Acquired AR generally occurs with endocarditis, rheumatic fever, or after surgical or transcatheter balloon valvotomy for valvar AS. AR is a progressive disease. The increased volume load on the LV initially results in compensatory hypertrophy, though the degree of hypertrophy becomes inadequate as regurgitation worsens (decompensated AR). Increasing LV volume associated with decreasing ventricular wall thickness results in increased afterload; eventually this increased afterload leads to myocardial damage and diminished contractility. In an effort to prevent irreversible myocardial damage, some echocardiographic guidelines have been established regarding the timing for intervention. In 2006, the ACC/AHA Task Force on Practice Guidelines recommended an end-diastolic diameter of 75 mm and an end-systolic diameter of 55 mm (as measured in transthoracic parasternal short axis views of the LV) as thresholds for intervention in asymptomatic adults with significant AR [65]. Comparable guidelines for the pediatric population have not yet been established.
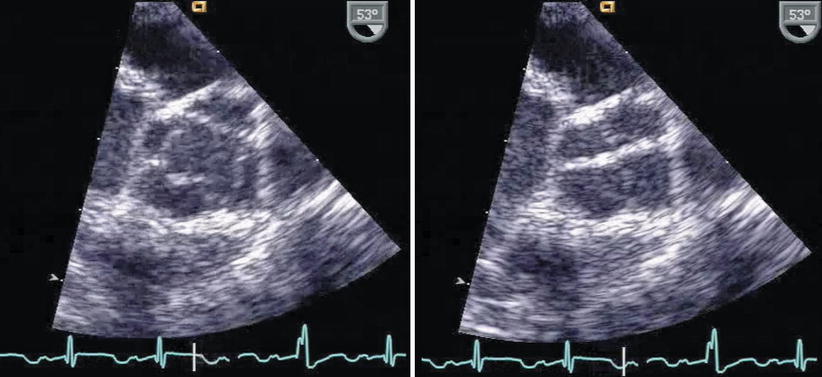
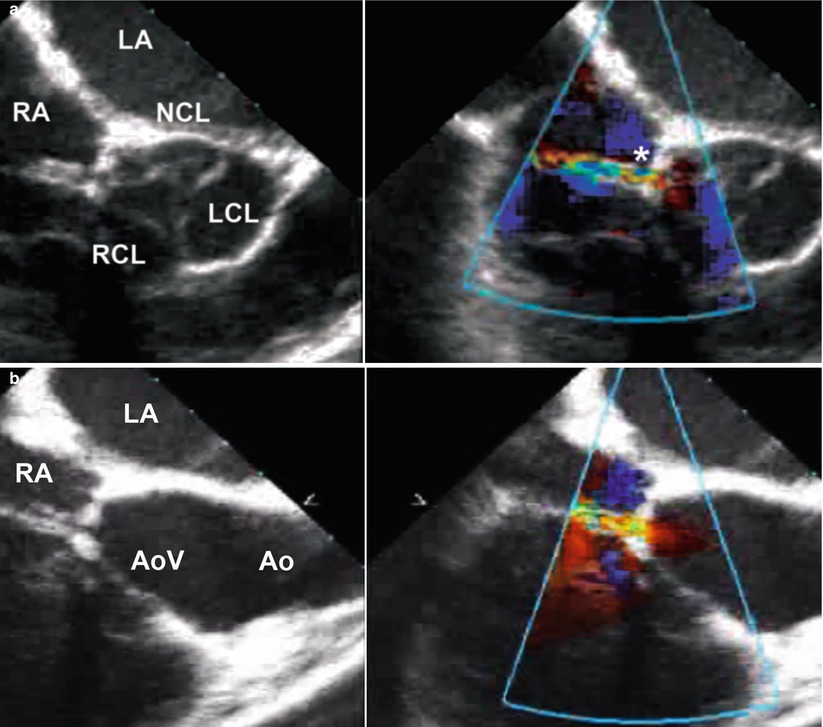

Fig. 11.15
Quadricuspid aortic valve as seen from the mid esophageal aortic valve short axis view, in systole (left figure) and diastole (right figure)

Fig. 11.16
(a) Mid esophageal aortic valve short axis view at 45° showing a short axis view of the aortic valve and a ruptured non-coronary sinus of Valsalva aneurysm (asterisk) into the right atrium; (b) Modified mid esophageal right ventricular inflow-outflow view at 90° showing the same ruptured of sinus of Valsalva aneurysm into the right atrium (Ao aorta, AoV aortic valve, LA left atrium, LCL left coronary leaflet, NCL non-coronary leaflet, RA right atrium, RCL right coronary leaflet)
The utility of transthoracic echocardiography in assessing the degree of AR and the appropriate timing for intervention has been discussed extensively [66]. Once a patient is in the operating room, TEE provides a highly accurate assessment of all types of AR lesions, whether it is annular dilation, leaflet prolapse (in the setting of a VSD), or leaflet deficiency as in a bicommissural aortic valve or in rheumatic heart disease [67]. This is especially important if surgical repair of the valve is a consideration as valves with bicommissural morphology and those after rheumatic fever tend to be better candidates for surgical repair [68]. Therefore, TEE should evaluate the AoV and aortic root morphology in multiple mid esophageal views. The ME AV SAX view at 30–45° will display abnormalities in AoV commissural morphology, discrepancies among the individual leaflet sizes, absence of one or more of the leaflets, dilation of a coronary artery in the setting of a significant coronary-cameral fistula into the LV, disruption of one or more AoV leaflets (flail segment) secondary to endocarditis or after valvotomy, or rupture of a sinus of Valsalva into one of the intracardiac chambers (Fig. 11.16a, Video 11.16a). The ME AV LAX view at ~120° and ME RV In-Out view between 60° and 90° will display abnormally thickened AoV leaflets after rheumatic fever, an aortico-left ventricular tunnel, or a ruptured sinus of Valsalva aneurysm (Fig. 11.16b, Video 11.16b). The location of the regurgitant jet should be assessed from multiple TEE windows, including the mid esophageal (ME AV SAX, ME AV LAX, ME LAX), deep transgastric (DTG LAX, DTG Sagittal), and transgastric (TG LAX) views. Regarding assessment of AR severity, a 2003 report from the American Society of Echocardiography task force on valvular regurgitation suggested that a vena contracta width for the regurgitant jet >0.6 cm and a ratio between the color flow jet width and the LVOT diameter >65 % represent severe AR [69]. Both of these measurements are best obtained from the ME AV LAX view. In keeping with these recommendations, the 2008 ACC/AHA task force [21] listed the following echocardiographic criteria for classification of AR:
Mild: Vena contracta width <0.3 cm, jet width/LVOT diameter <25 %
Moderate: Vena contracta width 0.3–0.6 cm, jet width/LVOT diameter 25–65 %
Severe: Vena contracta width >0.6 cm, jet width/LVOT diameter >65 %.
Other supportive data that can indicate at least moderate AR include prominent retrograde diastolic flow in the descending aorta, as noted by both color flow and pulsed wave Doppler [19, 20, 70]. This can best be evaluated using a combination of upper esophageal (UE Ao Arch LAX, SAX) and descending aorta long axis (Desc Ao LAX) and descending aortic short axis (Desc Ao SAX) views. There are few data on the utility of TEE to evaluate other indices of AR severity such as pressure half-time (which reflects the rate of equalization of aortic and LV diastolic pressures in the setting of AR), especially since this index can be affected by LV compliance, LV diastolic pressures, aortic compliance, and any therapy which changes ventricular afterload [70, 71]. In general, a pressure half-time > 500 ms is usually compatible with mild AR, while a value < 200 ms is consistent with severe AR [69]. Methods that have been discussed in other sources, but are rather difficult and time-consuming to perform in the intraoperative setting, include the calculation of effective regurgitant orifice area (EROA) by the flow convergence method (PISA) [69, 72]. If used, the following grading system (EROA in cm2) has been suggested: mild AR <0.10, moderate AR 0.10–0.30 severe AR >0.30 [21]. It should be noted that the degree of AR can be difficult to assess by TEE, particularly in the operating room in which changing hemodynamics and general anesthesia could artificially alter its severity. However, in a cohort of pediatric patients who had undergone aortic valve repair, Honjo et al. found that, using the jet width/LVOT criteria, there was reasonable agreement between the degree of AR found by intraoperative TEE and subsequently by pre-discharge transthoracic echocardiogram [73].
In addition to the evaluation of AR, LV size and function should be assessed qualitatively in the transgastric short axis views (TG Mid SAX, TG Basal SAX) at 0°. Associated abnormalities such as subvalvar AS, a VSD, and aortic dilation can be assessed in multiple views as discussed above.
Surgical intervention generally involves aortic valvuloplasty (occasionally involving the use of autologous pericardium), a Ross procedure (involving replacement of the aortic root with the patient’s pulmonary root and placement of a homograft from the RV to the pulmonary artery), or AoV replacement with a mechanical valve, bioprosthesis, or homograft valve. The postoperative TEE should exclude residual LVOT obstruction using the transgastric LAX and deep transgastric views: DTG LAX at 0–30°, and DTG Sagittal view at ~90°. Residual AR can be evaluated in multiple mid esophageal, transgastric, and deep transgastric views. After aortic valvuloplasty, a maximum instantaneous gradient >45 mmHg or degree of AR ≥ moderate are considered indications to return to cardiopulmonary bypass for a repeat AoV repair or replacement [74]. After a Ross procedure, any neo-AR is a highly sensitive predictor of ≥ moderate neo-AR at the time of hospital discharge [75] If a prosthetic aortic valve is present, symmetric motion of the valve leaflets can usually be seen in the mid esophageal views, particularly the ME AV SAX and ME AV LAX views (Fig. 11.17, Video 11.17), though this can become difficult secondary to acoustic interference from the prosthetic valve annular ring. The transgastric and deep transgastric views are helpful here: since they visualize the valve from the left ventricular aspect, they avoid shadowing and reverberation artifacts, allowing evaluation of the “upstream” portion of the prosthetic valve and leaflet motion. Doppler evaluation of the prosthetic valve should be performed from the transgastric or deep transgastric views, as described above and discussed in Chap. 16.