Everolimus-eluting bioresorbable vascular scaffold (BVS) implantation in chronic total occlusion (CTO) could provide theoretical advantages at follow-up compared with metallic stents. This study aimed to assess the feasibility of BVS use for the percutaneous treatment of CTO by analyzing clinical outcomes and patency at midterm follow-up. From February 2013 to June 2014, 42 patients with 46 CTOs were treated by BVS implantation. Once the guidewire reached the distal lumen, all the occluded segments were predilated. Postdilation was performed in all patients. A multislice computed tomography was scheduled for all patients at 6 months. The mean age was 58 ± 9 years, 41 (98%) were men and 14 (33%) diabetic. The target vessel was predominantly the left anterior descending artery (22, 48%). According to the Japanese-CTO score, 21 CTOs (46%) were difficult or very difficult. Most cases were treated with an anterograde strategy (34 lesions, 74%). A hybrid procedure with a drug-eluting stent at the distal segment was the applied treatment in 7 CTOs (15%). The mean scaffold length was 43 ± 21 mm. Technical success was achieved in 45 lesions (98%), and 1 patient (2.4%) presented a non-Q periprocedural myocardial infarction. Re-evaluation was obtained in all patients at 6 ± 1 months. Two re-occlusions and a focal restenosis were identified. After 13 ± 5 months of follow-up, there were 2 repeat revascularizations (4.8%). Neither death nor myocardial infarction was documented. In conclusion, BVS for CTO seems to be an interesting strategy with a high rate of technical success and low rate of cardiac events at midterm follow-up in selected patients.
Coronary chronic total occlusion (CTO) currently represents the most challenging type of lesion for percutaneous coronary intervention (PCI). Although technological improvements and the development of specific skills by the operators have considerably increased the success rate, it continues to be low compared with non-CTO PCI. In addition, after recanalization, a long coronary segment must usually be stented. It is known that the stented length is a strong predictor of events at follow-up, such as stent thrombosis and need for repeat revascularization. Additionally, the permanent caging of the artery inhibits the recovery of the physiological proprieties of the vessel. Everolimus-eluting bioresorbable vascular scaffold (BVS) (Absorb; Abbott Vascular, Santa Clara, California) implantation in this type of lesion may be an attractive alternative. The reabsorption of the device could provide theoretical advantages at long-term follow-up compared with metallic drug-eluting stents (DES). The present study aimed to assess the feasibility of BVS for the percutaneous treatment of CTO and to analyze the clinical outcomes and BVS patency by multislice computed tomography scan (MSCT) at midterm follow-up.
Methods
From February 2013 to June 2014, 121 CTOs in 116 patients were recanalized and treated percutaneously in our center. Among them, 42 patients with 46 CTOs were successfully treated by BVS implantation, and they constitute the study group. Whether to implant a BVS was based on clinical data and anatomical characteristics of the lesion and was taken during the procedure. Exclusion criteria for treating with BVS were as follows: patients >70 years, vessel diameter >4 mm, very heavily calcified lesions together with extreme tortuosity, and patients with a contraindication to 1 year of dual antiplatelet therapy. Concurrent DES implantation was allowed at the operator’s discretion. Written informed consent for treatment and for data analysis was obtained from all patients.
The complexity of the lesion and difficulty of crossing was graded according to the Japanese-CTO score. The decision of an antegrade or retrograde approach was taken by the operator after a thorough study of the CTO anatomy using simultaneous double injection when applicable. Once the guidewire reached the distal lumen, all the occluded segments were painstakingly predilated with balloons of increasing diameter while testing the uniform expansion of them. After BVS implantation, postdilation using noncompliant balloons with a maximum diameter 0.5 mm more than the BVS diameter was systematically performed. Intravascular ultrasound was used in some procedures to facilitate the access to the guidewire to the true lumen ( Figure 1 ) or to determine the vessel diameter. Optical coherence tomography was performed when the BVS platform was manipulated or some complication was suspected. All patients were pretreated with dual antiplatelet medication. In the hemodynamic laboratory, weight-adjusted heparin was administered to maintain activated clotting time for >300 seconds. After the procedure, the patients received aspirin 100 mg/day indefinitely and a maintenance dose of clopidogrel (75 mg/day), prasugrel (10 mg/day), or ticagrelor (90 mg twice daily) for at least 12 months. In all patients, serial determinations of the troponin I and creatine kinase levels were performed before and every 6 hours after the procedure for the first 24 hours.
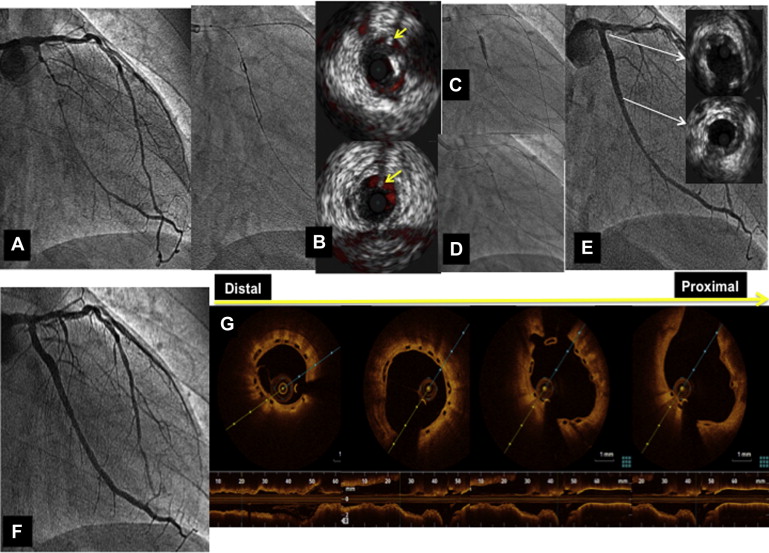
CTO was defined as complete obstruction of the vessel with Thrombolysis In Myocardial Infarction flow grade 0 through the affected segment of >3 months estimated duration. Technical success was considered when a patent vessel with <30% residual stenosis and a Thrombolysis In Myocardial Infarction flow grade 3 was achieved. Major adverse cardiac events (MACEs) were defined as a composite of cardiac death, myocardial infarction (MI), and target lesion revascularization. Periprocedural MI was defined according to the new definition of clinically relevant MI after coronary revascularization, and scaffold thrombosis was defined according to the Academic Research Consortium criteria.
After the treatment, the patients were monitored closely by scheduled visits and phone calls. To study the patency of the treated CTO at follow-up, an MSCT was scheduled for all patients 6 months after the treatment. A new cardiac catheterization was recommended in the presence of clinical recurrence.
Descriptive analyses were used. Discrete variables are presented as counts and percentages, whereas continuous data are presented as the mean ± SD (interquartile range 25 to 75). All analyses were performed using SPSS 20.0.0 software (IBM).
Results
Baseline clinical and angiographic data are listed in Tables 1 and 2 . All patients had stable angina despite optimal medical treatment. Unlike the patients treated exclusively with DES, those treated with BVS were younger (58 ± 9 vs 67 ± 8; p <0.05) and with a lower prevalence of diabetes mellitus (14, 33% vs 39, 53%; p <0.05). According to the Japanese-CTO score of complexity, 59 CTOs (78%) were classified as difficult or very difficult in the DES group, whereas in the BVS group 21 CTOs (46%) were difficult or very difficult (p <0.05).
| Variables | Patients (n=42) |
|---|---|
| Age (years) | 58±9 |
| Men | 41 (98%) |
| Diabetes mellitus | 14 (33%) |
| Hypertension ∗ | 24 (57%) |
| Hypercholesterolemia † | 27 (64%) |
| Current smoker | 8 (19%) |
| Prior myocardial infarction | 12 (28%) |
| Prior percutaneous coronary intervention | 15 (36%) |
∗ Repeated systemic blood pressure measurements >140/90 mmHg or treatment with antihypertensive drugs for a known diagnosis of hypertension.
† Baseline cholesterol > 200 mg/dl or LDL-cholesterol >130 mg/dl or previously diagnosed and treated hypercholesterolemia.
| Variables | Patients (n=42)/Lesions (n=46) |
|---|---|
| LV ejection fraction (%) | 54±8 |
| Target coronary artery: | |
| Left anterior descending artery | 22 (48%) |
| Right | 13 (28%) |
| Left circumflex | 11 (24%) |
| Multivessel disease | 28 (67%) |
| CTO complexity (J-CTO score): | |
| Easy | 6 (13%) |
| Intermediate | 19 (41%) |
| Difficult | 14 (31%) |
| Very difficult | 7 (15%) |
| Estimated duration of the CTO (months) | 35±42 |
| Main vessel reference (mm) | 3.03±0.4 |
| Lesion length (mm) | 35±19 |
| Minimal lumen diameter (mm) | 0±0 |
| Diameter stenosis pre (%) | 100±0 |
| Minimal lumen diameter post (mm) | 2.7±0.4 |
| Diameter stenosis post (%) | 11±6 |
Table 3 summarizes the procedural characteristics. In 34 lesions (74%), an antegrade approach with a microcatheter and different dedicated wires was the strategy used to cross the CTO, whereas in 12 (26%), a retrograde approach was needed. In these cases, reverse controlled antegrade and retrograde subintimal tracking (it consists on inflating a balloon on the antegrade wire to enlarge the antegrade subintimal/subadventitial space, whereas the retrograde wire is then manipulated to enter the enlarged antegrade space) was the technique used in most of the procedures. Twenty-two recanalized CTOs (48%) were treated with 1 single BVS, 18 (39%) were treated with 2, and 6 (13%) received 3 BVSs.
| Variables | n=46 |
|---|---|
| Femoral approach | 46 (100%) |
| Seath 8Fr | 46 (100%) |
| Double injection | 26 (56%) |
| Strategy: | 0±0 |
| Antegrade | 34 (74%) |
| Retrograde | 12 (26%) |
| Predilation | 46 (100%) |
| Bioresorbable vascular scaffold diameter (mm) | 3.03±0.38 |
| Scaffold length (mm) | 43±21 |
| Number of bioresorbable vascular scaffolds/lesion | 2.6±1.9 |
| Hybrid strategy | 7 (15%) |
| Scaffold postdilation | 46 (100%) |
| Bifurcation lesion involved: | 11 (24%) |
| Side branch predilation+Main vessel scaffold | 5 (45%) |
| Isolated side branch postdilation | 6 (55%) |
| Intravascular ultrasound: | 5 (11%) |
| To access to the true lumen | 1 (20%) |
| To determine vessel size | 4 (80%) |
| Optical coherence tomography | 14 (30%) |
Regarding in-hospital outcomes, technical success was achieved in 45 of 46 lesions (98%). In 1 patient with a very complex CTO (J-CTO score >3 points), a residual distal lesion >30% was left because of the impossibility of passing through the several BVSs. No major complications related to the procedure were registered. One patient (2.4%) presented with significant troponin elevation in the range associated with a non-Q periprocedural MI. No other in-hospital adverse clinical events were recorded, and all patients were discharged free of symptoms.
At midterm follow-up (mean: 13 ± 5 months, median: 12, IQ 25–75 : 9.75 to 16 months), the overall MACE rate was 4.8% because of 2 repeat revascularizations. Neither death nor AMI were documented. Re-evaluation by coronary MSCT scan ( Figures 2 and 3 ) or angiography ( Figure 1 ) in cases of clinical recurrence was obtained in all patients at 6 ± 1 months after the treatment. An asymptomatic complete scaffold re-occlusion was detected at 6 months in a patient who had a significant residual stenosis that was not treated. Two patients had restenosis, which was focal in one of them and treated by balloon dilation, whereas in the other a re-occlusion was observed. That re-occlusion was again recanalized, and a DES was implanted. The rest of the treated CTOs continued being open, without restenosis and the patients remain free of symptoms. In the DES group, the MACE rate at follow-up was 8%. Two patients (2.7%) died from heart failure and 4 (5.4%) required repeat revascularization.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


