Patients with ST-segment elevation myocardial infarction (STEMI) admitted during nonregular working hours (off-hours) have been reported to have greater mortality than those admitted during regular working hours (on-hours), perhaps because of the lower availability of catheterization laboratory services and longer door-to-balloon times. This might not be the case, however, for hospital centers in which primary percutaneous coronary intervention (PCI) is invariably performed. We conducted a substudy using the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction study data to determine whether the STEMI arrival time was associated with differing clinical outcomes. We identified all patients with STEMI admitted to a PCI-capable hospital who underwent primary PCI. Patients presenting during on-hours were compared to those presenting during off-hours. The primary outcome of death, major adverse cardiovascular events, and net adverse clinical events was examined. We identified 2,440 patients (1,205 [49%] on-hours and 1,235 [51%] off-hours). Similar baseline characteristics were observed. The off-hour patients had a significantly longer door-to-balloon time (92 vs 75 minutes; p <0.0001) and total ischemic time (209 vs 194 minutes; p <0.0001). Despite these differences, the risk-adjusted all-cause mortality, major adverse cardiovascular events, and net adverse clinical events rates were similar for both groups during the in-hospital, 1-year, and 3-year follow-up. In conclusion, patients with STEMI presenting to primary PCI hospitals during off-hours might have slightly longer delays to revascularization; however, they experienced similar short- and long-term survival and clinical outcomes as those arriving during on-hours.
Greater mortality rates have been reported among patients admitted with acute myocardial infarction during nonregular working hours (i.e., nights, weekends, and holidays). A few of the proposed explanations have included the lower use of primary percutaneous coronary intervention (PCI), fatigue and operator experience, circadian variation in myocardial perfusion, and increased reperfusion time during off-hours. The data published to date remain controversial, because other studies have found no difference in ST-segment elevation myocardial infarction (STEMI) outcome during off-hours. Most of the available data, however, were derived from uncontrolled retrospective registries with inconsistent and unadjudicated follow-up data of variable duration. We used the large database of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, a prospective cohort with clearly defined STEMI, to determine whether the arrival time was associated with worse short- and long-term outcomes in those undergoing primary PCI.
Methods
The HORIZONS-AMI trial was a prospective, open-label, dual-arm, factorial, randomized, multicenter trial in which bivalirudin alone was compared to heparin plus a glycoprotein IIb/IIIa inhibitor, and paclitaxel-eluting stents were compared to bare metal stents in patients with STEMI undergoing primary PCI. The study enrolled 3,602 patients from March 2005 to May 2007 among 123 hospitals in 11 countries. Consecutive patients aged ≥18 years who presented within 12 hours after the onset of symptoms and who had STEMI of ≥1 mm in ≥2 contiguous electrocardiographic leads, new left bundle branch block, or true posterior myocardial infarction were considered for enrollment. The exclusion criteria have been previously reported and included contraindications to the study medications, bleeding diathesis, planned surgery within 6 months that would require interruption of thienopyridine therapy, previous (within 30 days) stenting, or life expectancy <1 year. Patients were not excluded because of cardiogenic shock, previous cardiac arrest, or other high-risk conditions. The institutional review board or ethics committee at each participating hospital approved the study, and all patients gave written informed consent. Patients enrolled in this trial had presented either directly to a tertiary PCI hospital or to a non-PCI hospital and were subsequently transferred to a PCI hospital. Informed consent was obtained at the study PCI hospital, where patients were randomly assigned using a computerized, interactive, voice-response system in a 1:1 ratio to treatment with unfractionated heparin plus a glycoprotein IIb/IIIa inhibitor or bivalirudin alone. After coronary angiography, 3,011 patients with lesions eligible for stenting were subsequently randomized in a 3:1 ratio to either a slow rate-release paclitaxel-eluting stent (TAXUS Express, Boston Scientific, Natick, Massachusetts) or an otherwise identical uncoated bare metal stent (Express, Boston Scientific). Clinical follow-up examinations for all patients undergoing primary randomization was performed at 30 days, 6 and 12 months, and then yearly for 5 years.
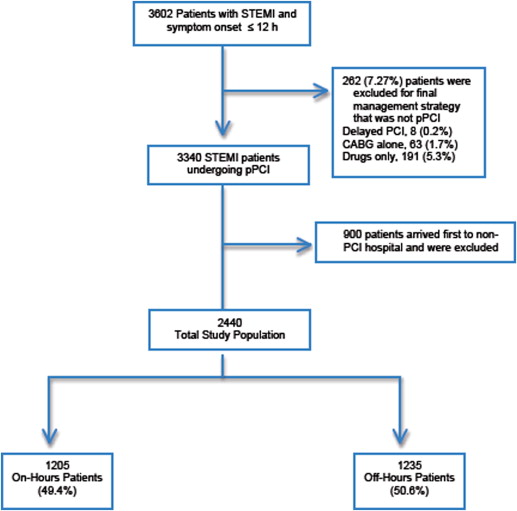
Of the HORIZONS-AMI patients who underwent primary PCI (n = 3,340, 92.7%), only those presenting directly to a PCI-capable hospital were included in the present analysis (n = 2,440; Figure 1 ). Patients arriving to non-PCI hospitals who were subsequently transferred for primary PCI were excluded from the present analysis to avoid a potential referral bias. All study patients were stratified according to the day and time of arrival into on-hours and off-hours groups. On-hours was defined as weekdays from 8:00 a.m. to 5:00 p.m . Off-hours was defined as weekdays from 5:00 p.m. to 8:00 a.m. and all weekends and holidays (according to each country’s calendar holidays). The catheterization laboratory response teams remained off site in all participating hospitals during off-hours and were called in emergently for each individual STEMI case according to each hospital’s STEMI alert protocol.
All end point events were adjudicated through the use of original source documentation by an independent committee who was unaware of the treatment allocation. The primary end points of the present study were death, major adverse cardiovascular events (MACE), and net adverse clinical events during the in-hospital, 1-year, and 3-year follow-up points. MACE was defined as death, reinfarction, stroke, or unplanned target vessel revascularization for ischemia. Net adverse clinical events included the composite of MACE and major bleeding not related to coronary artery bypass grafting. Major bleeding was considered as any of the following: intracranial, intraocular, or peritoneal bleeding; access site hemorrhage requiring surgery or a radiologic or interventional procedure; hematoma ≥5 cm in diameter at the arterial access site; a decrease in the hemoglobin level of ≥4 g/dl without an overt bleeding source or ≥3 g/dl with an overt source; and bleeding that required transfusion or reoperation. Bleeding was also assessed and adjudicated on the basis of the Thrombolysis In Myocardial Infarction and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) scales. A subanalysis of the total ischemic time was performed to assess for differences between the patients treated on- and off-hours.
All study patients were given 325 mg oral or 500 mg intravenous aspirin in the emergency room, after which 300 to 325 mg was given daily during their hospitalization, followed by 75 to 81 mg/day indefinitely. A loading dose of clopidogrel (either 300 or 600 mg) or ticlopidine (500 mg), in case of clopidogrel allergy, was administered before catheterization, followed by 75 mg/day orally for ≥6 months. Heparin plus a glycoprotein IIb/IIIa inhibitor or bivalirudin alone was administered according to the randomization protocol of the original HORIZONS-AMI study design. Heparin was administered as an intravenous bolus of 60 IU/kg, with subsequent boluses targeted to an activated clotting time of 200 to 250 seconds. Bivalirudin was administered as an intravenous bolus of 0.75 mg/kg, followed by an infusion of 1.75 mg/kg/hour. Both antithrombin agents were discontinued, as specified by the protocol, at the completion of angiography or PCI but could be continued at low doses, if clinically indicated. A glycoprotein IIb/IIIa inhibitor was routinely administered before PCI in the control group and was administered in the bivalirudin group only in patients with no reflow or with large thrombus burden during PCI. Either abciximab (a bolus of 0.25 μg/kg followed by an infusion of 0.125 μg/kg/min; maximum dose 10 μg/min) or double bolus of eptifibatide (a bolus of 180 μg/kg followed by an infusion of 2.0 μg/kg/min, with a second bolus given 10 minutes after the first; no maximum dose was prespecified), adjusted for renal impairment according to the label, was permitted at the discretion of the investigator and was continued for 12 hours for abciximab or 12 to 18 hours for eptifibatide. After the establishment of a patent infarct vessel, eligible patients undergoing primary PCI were randomized to a paclitaxel-eluting stent or bare metal stent, as described. The study investigators were given the discretion to choose the vascular access site, guidewire type, thrombectomy catheter, and all other additional procedure-related techniques.
The following points were prospectively obtained: (1) time of symptom onset, available for 2,416 cases (99%), (2) time of arrival to the PCI hospital (door time), available for 2,368 patients (97%), (3) catheterization laboratory arrival time, available for 2,434 patients (99%), and (4) first balloon inflation time, available for 2,177 patients (89%). These data were used to determine the following 5 intervals: (1) symptom-to-door time, (2) door-to-balloon (DTB) time, (3) total ischemic time (TIT), equal to the sum of the symptom-to-door and DTB times, available for 2,088 patients (86%), (4) total procedure time, and (5) hospital length of stay.
Categorical outcomes were compared using the chi-square test or Fisher’s exact test. Continuous data are reported as the median and interquartile range and were compared using the Wilcoxon rank sum test. Differences in mortality, MACE, and net adverse clinical events between the on- and off-hours primary PCI patients during the follow-up period were assessed using the Kaplan-Meier method and compared using the log-rank test. Multivariate logistic regression analysis was conducted to evaluate the adjusted effect estimates associated with presentation during off-hours for primary PCI. For the analysis, the prespecified end point variables of death, MACE, and net adverse clinical events were considered the dependent variables of interest. The following variables were included in the multivariate regression analysis models: age, gender, Killip class II to IV, baseline Thrombolysis In Myocardial Infarction flow of 0 or 1, creatinine level, hemoglobin level, 10% decrease in left ventricular ejection fraction, randomization to bivalirudin, history of diabetes mellitus, smoking history, DTB, TIT, left anterior descending infarct artery, and administration of clopidogrel.
Results
Of the 2,440 patients included in the present substudy, 1,205 (49%) arrived to a PCI hospital during on-hours and 1,235 (51%) during off-hours. The median age was 60.2 years (interquartile range 52.4 to 69.7), with 76.6% men and 16.8% diabetics. The baseline demographic characteristics are listed in Table 1 . The on-hours patients had, on average, a greater prevalence of previous angina (p = 0.008), and those presenting during off-hours were more likely to report a history of hyperlipidemia (p = 0.043) and to be of Killip class II-IV (p = 0.01). The study medications, angiographic findings, and procedure characteristics are summarized in Table 2 . Patients in the on-hours group were more likely to receive a 600-mg loading dose of clopidogrel (on-hours 66.4% vs off-hours 61.8%; p = 0.021). The culprit artery was the right coronary artery in 41.7% and left anterior descending artery in 40.9%; 4.2% of patients had multiple vessels treated. The PCI procedures during on-hours were characterized by a greater use of aspiration thrombectomy catheters (on-hours 14.4% vs off-hours 10.4%; p = 0.004). Stents were implanted in ≤93.4% of patients; 74.9% were paclitaxel-eluting stents and 25.1% were bare metal stents, with symmetric distribution between the on- and off-hour groups.
| Variable | On-hours (n = 1,205) | Off-hours (n = 1,235) | p Value |
|---|---|---|---|
| Age (yrs) | 0.189 | ||
| Median | 60.1 | 59.4 | |
| Interquartile range | 52.6–69.9 | 51.9–69.6 | |
| Men | 913 (75.8%) | 957 (77.5%) | 0.175 |
| Body mass index (kg/m 2 ) | 0.771 | ||
| Median | 26.9 | 27.1 | |
| Interquartile range | 24.4–30.1 | 24.6–30.4 | |
| Diabetes mellitus, total | 189 (15.7%) | 220 (17.8%) | 0.242 |
| Insulin-dependent diabetes mellitus | 62 (5.1%) | 49 (4.0%) | 0.594 |
| Hypertension | 612 (50.8%) | 664 (53.8%) | 0.816 |
| Hyperlipidemia | 507 (42.1%) | 570 (46.2%) | 0.043 |
| Current or past smoker | 740 (61.8%) | 792 (64.4%) | 0.554 |
| Previous myocardial infarction | 140 (11.6%) | 145 (11.7%) | 0.422 |
| Previous percutaneous coronary intervention | 153 (12.7%) | 150 (12.1%) | 0.331 |
| Previous coronary artery bypass grafting | 38 (3.2%) | 38 (3.1%) | 0.307 |
| Previous angina pectoris | 253 (21.0%) | 229 (18.5%) | 0.008 |
| Heart failure | 34 (2.8%) | 37 (3.0%) | 0.088 |
| New York Heart Association class I | 8 (0.7%) | 9 (0.7%) | 0.847 |
| New York Heart Association class II | 20 (1.7%) | 21 (1.7%) | 0.938 |
| New York Heart Association class III | 4 (0.3%) | 5 (0.4%) | 1.000 |
| New York Heart Association class IV | 1 (0.1%) | 1 (0.1%) | 1.000 |
| Killip class II-IV | 84 (7.0%) | 124 (10.0%) | 0.010 |
| Left ventricular ejection fraction (%) | 0.635 | ||
| Median | 50 | 50 | |
| Interquartile range | 43–60 | 43–60 | |
| Family history of premature coronary artery disease | 321 (26.6%) | 373 (30.2%) | 0.051 |
| History of cardiac rhythm/rate disturbance | 44 (3.7%) | 32 (2.6%) | 0.195 |
| Peripheral vascular disease | 56 (4.6%) | 47 (3.8%) | 0.215 |
| History of thrombocytopenia | 6 (0.5) | 1 (0.1) | 0.369 |
| Renal insufficiency | 41 (3.4) | 36 (2.9) | 0.891 |
| Variable | On-hours (n = 1,205) | Off-hours (n = 1,235) | p Value |
|---|---|---|---|
| Aspirin | |||
| Before arriving to percutaneous coronary intervention hospital | 319/1,202 (26.5%) | 342/1,234 (27.7%) | 0.514 |
| During index hospitalization | 1,199/1,202 (99.8%) | 1,232/1,235 (99.8%) | 1.000 |
| At discharge | 1,150/1,179 (97.5%) | 1,170/1,199 (97.6%) | 0.948 |
| Bivalirudin | 605 (50.2%) | 629 (50.9%) | 0.721 |
| Heparin plus glycoprotein IIb/IIIa inhibitor | 600 (49.8%) | 606 (49.1%) | 0.721 |
| Clopidogrel | |||
| Before arriving to percutaneous coronary intervention hospital | 43/1,201 (3.6%) | 41/1,235 (3.3%) | 0.725 |
| During index hospitalization | 1,181/1,202 (98.3%) | 1,211/1,234 (98.1%) | 0.829 |
| Loading dose 300 mg | 376/1,162 (32.4%) | 436/1,193 (36.5%) | 0.033 |
| Loading dose 600 mg | 771/1,162 (66.4%) | 737/1,193 (61.8%) | 0.021 |
| At discharge | 1,079/1,179 (91.5%) | 1,107/1,199 (92.3%) | 0.469 |
| Culprit coronary artery | |||
| Left anterior descending | 486/1,168 (41.6%) | 506/1,255 (40.3%) | 0.519 |
| Left circumflex | 178/1,168 (15.2%) | 202/1,255 (16.1%) | 0.563 |
| Right | 484/1,168 (41.4%) | 526/1,255 (41.9%) | 0.813 |
| Saphenous vein graft | 12/1,168 (1.0%) | 12/1,255 (1.0%) | 0.860 |
| Left main | 8/1,168 (0.7%) | 8/1,255 (0.6%) | 0.885 |
| Other ∗ | 0/1,168 (0.0%) | 1/1,255 (0.1%) | 1.000 |
| Thrombolysis In Myocardial Infarction flow | |||
| Before percutaneous coronary intervention | |||
| 0–1 | 764/1,167 (65.5%) | 837/1,253 (66.8%) | 0.489 |
| 2 | 196/1,167 (16.8%) | 187/1,253 (14.9%) | 0.208 |
| 3 | 207/1,167 (17.7%) | 229/1,253 (13.8%) | 0.731 |
| After percutaneous coronary intervention | |||
| 0–1 | 25/1,166 (2.1%) | 30/1,254 (2.4%) | 0.682 |
| 2 | 68/1,166 (5.8%) | 69/1,254 (5.5%) | 0.726 |
| 3 | 1,073/1,166 (92.0%) | 1,155/1,254 (92.1%) | 0.941 |
| Multiple lesions treated | 106/1,084 (9.8%) | 131/1,123 (11.7%) | 0.152 |
| Percutaneous transluminal coronary angioplasty only | 48/1,093 (4.4%) | 44/1,130 (3.9%) | 0.556 |
| Patients with ≥1 stent implanted | 1,042/1,119 (93.1%) | 1,074/1,146 (93.7%) | 0.566 |
| Drug-eluting stent | 742/991 (74.9%) | 772/1,030 (75.0%) | 0.968 |
| Bare metal stent | 249/991 (25.1%) | 258/1,030 (25.0%) | 0.968 |
| Any side branch lesion treated | 85/1,119 (7.6%) | 82/1,145 (7.2%) | 0.693 |
| Any closure device | 302/1,059 (28.5%) | 337/1,092 (30.9%) | 0.234 |
| Any aspiration catheter | 158/1,097 (14.4%) | 118/1,136 (10.4%) | 0.004 |
| Any thrombectomy device | 13/1,095 (1.2%) | 12/1,133 (1.1%) | 0.774 |
| Fluoroscopy time (min) | 0.073 | ||
| Mean | 11 | 12 | |
| Interquartile range | 7–17 | 8–18 | |
| Contrast volume (ml) | 0.209 | ||
| Mean | 220 | 230 | |
| Interquartile range | 175–290 | 180–290 | |
| Peak activated clotting time (s) | 0.140 | ||
| Mean | 310 | 305 | |
| Interquartile range | 250–398 | 250–380 |
The intervals recorded during the study are summarized in Table 3 . The symptom-to-door time (p = 0.66) and total procedure time (p = 0.11) were similar between the 2 groups; however, the patients with STEMI who presented during off-hours had significantly longer delays and prolonged “emergency room to catheterization laboratory arrival time” (53 vs 47 minutes; p = 0.037), DTB time (92 vs 75 minutes; p <0.0001), and TIT (209 vs 194 minutes; p <0.0001). No differences were observed in the median hospital length of stay (p = 0.26).
| On-hours (n = 1,205) | Off-hours (n = 1,235) | Combined (n = 2,440) | p Value | |
|---|---|---|---|---|
| Symptom-to-door time (min) | 110 (70–195) | 117 (65–195) | 114 (68–195) | 0.656 |
| Door-to-balloon time (min) ∗ | 75 (54–100) | 92 (69–118) | 83 (61–110) | <0.0001 |
| Total ischemic time (min) † | 194 (141–289) | 209 (158–309) | 200 (150–300) | <0.0001 |
| Total procedure time (min) ‡ | 40 (27–56) | 40 (29–57) | 40 (28–56) | 0.109 |
| Hospital length of stay (days) § | 5 (3–7) | 5 (4–7) | 5 (3–7) | 0.258 |
∗ Interval from PCI hospital arrival to first balloon inflation.
† Interval from symptom onset to first balloon inflation.
‡ Interval between first and last angiogram.
The mean death rate for the study population was 2.4%, 4.2%, and 6.5% during the in-hospital, 1-year, and 3-year follow-up period, respectively ( Table 4 ). The patients with STEMI admitted during off-hours had comparable adjudicated rates of death, MACE, and net adverse clinical events at both short- and long-term follow-up. A greater in-hospital reinfarction rate was observed in the off-hours group (p = 0.021); however, the reinfarction rates were not significantly different at 1 and 3 years of follow-up. The Kaplan-Meier survival curves for the 3 primary end points are shown for the 36-month study period ( Figure 2 ).
| In-hospital Outcome | 1-y Outcome | 3-y Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|
| On-hours (n = 1,205) | Off-hours (n = 1,235) | p Value | On-hours (n = 1,205) | Off-hours (n = 1,235) | p Value | On-hours (n = 1,205) | Off-hours (n = 1,235) | p Value | |
| Major adverse cardiovascular events ∗ | 45 (3.7%) | 57 (4.6%) | 0.277 | 133 (11.3%) | 149 (12.3%) | 0.482 | 252 (21.9%) | 264 (22.1%) | 0.830 |
| Death | 23 (1.9%) | 35 (2.8%) | 0.134 | 43 (3.6%) | 56 (4.6%) | 0.238 | 75 (6.5%) | 78 (6.5%) | 0.934 |
| Reinfarction | 7 (0.6%) | 19 (1.5%) | 0.021 | 41 (3.6%) | 46 (3.9%) | 0.664 | 83 (7.5%) | 82 (7.1%) | 0.803 |
| Target vessel revascularization for ischemia | 24 (2.0%) | 22 (1.8%) | 0.703 | 78 (6.8%) | 81 (6.9%) | 0.979 | 147 (13.1%) | 160 (13.9%) | 0.622 |
| Any stroke | 3 (0.2%) | 2 (0.2%) | 0.684 | 10 (0.9%) | 10 (0.8%) | 0.951 | 21 (1.9%) | 17 (1.5%) | 0.465 |
| Net adverse clinical events † | 114 (9.5%) | 123 (10.0%) | 0.677 | 201 (17.1%) | 213 (17.4%) | 0.798 | 311 (26.8%) | 328 (27.3%) | 0.784 |
| Major bleeding (noncoronary artery bypass graft) | 81 (6.7%) | 84 (6.8%) | 0.938 | 91 (7.6%) | 93 (7.6%) | 0.976 | 101 (8.6%) | 107 (8.9%) | 0.821 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


