Fig. 15.1
Echocardiographic appearances of the heart before (pre-PTE; top) and after (post-PTE; bottom) operation. Note the shift of the intraventricular septum toward the left in systole before the operation (top left), together with the relatively small left atrial and left ventricular chambers. After the operation, the septum is restored to its normal geometry, and the massive enlargement of the right atrium and right ventricle has resolved
The ventilation-perfusion lung scan is the fundamental test for establishing the diagnosis of unresolved pulmonary thromboembolism. An entirely normal lung scan excludes the diagnosis of both acute or chronic, unresolved thromboembolism. The usual lung scan pattern in most patients with primary pulmonary hypertension either is relatively normal or shows a diffuse non-uniform perfusion. When subsegmental or larger perfusion defects are noted on the scan, even when matched with ventilatory defects, pulmonary angiography is appropriate to confirm or rule out thromboembolic disease. It is important to note that any patient with unexplained dyspnea should be worked up for pulmonary hypertension, and any patient with a diagnosis of pulmonary hypertension should undergo a V/Q scan.
Currently, pulmonary angiography still remains the gold standard for diagnosis of CTEPH, however with the advent of high resolution scans, and magnetic resonance imaging, more and more centers rely on the diagnostic power and the non-invasive nature of these tests to confirm the diagnosis. Organized thromboembolic lesions do not have the appearance of the intravascular filling defects seen with acute pulmonary emboli, and experience is essential for the proper interpretation of pulmonary angiograms in patients with unresolved, chronic embolic disease. Organized thrombi appear as unusual filling defects, webs, or bands, or completely thrombosed vessels that may resemble congenital absence of the vessel [11] (Fig. 15.2). In addition to pulmonary angiography, patients over 45 undergo coronary arteriography and other cardiac investigation as necessary. If significant disease is found, additional cardiac surgery is performed at the time of pulmonary thromboendarterectomy.
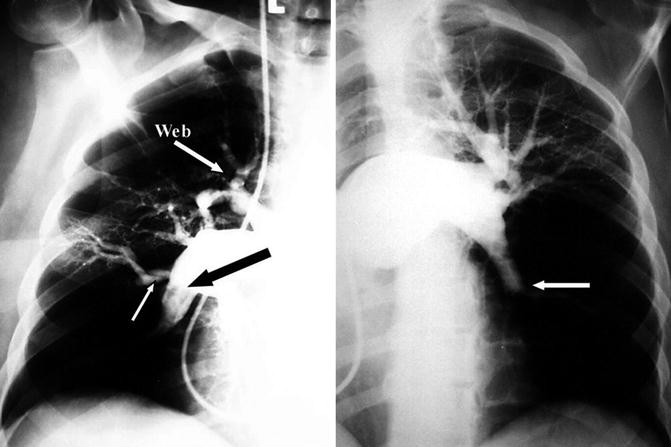

Fig. 15.2
Pulmonary angiogram in a patient with chronic thromboembolic pulmonary hypertension. Please note the extensive areas of hypoperfusion as a result of complete occlusion, as well as luminal irregularities, webs, bands, and pouches, shown by arrows
In recent years higher resolution helical computed tomography (CT) scans of the chest have been used more frequently in diagnosis of pulmonary thromboembolic disease. Presence of large clots in lobar or segmental vessels generally confirms the diagnosis. CT features of chronic thromboembolic pulmonary hypertension include evidence of organized thrombus lining the pulmonary vessels in an eccentric fashion, enlargement of the right ventricle and the central arteries, variation in size of segmental arteries, and parenchymal changes characteristic of pulmonary infarction. In addition, in rare situations where there are concerns of external compression, or occlusion of main pulmonary arteries are present, CT scans can be helpful in differentiating thromboembolic disease from other causes such as mediastinal fibrosis, lymph nodes, or tumors. In the current era of multi-detector CT scans, the resolution of the pulmonary arteries is much better and it is possible that this diagnostic modality may eventually replace pulmonary angiography as the gold standard in diagnosing CTEPH and in planning the surgical treatment once the diagnosis is made. In addition, the volumetric assessment of the right ventricle can be more accurately performed with multi-detector CT. It is important to remember, however, that the diagnosis of CTEPH by CT scans is difficult and requires experienced radiologists with expertise in this area and currently remains an adjunct to pre-operative planning.
Medical Treatment
There is no curative role for medical management of these patients and at best it is palliative. There are a number of new pulmonary vasodilators that are now available for the treatment of the pulmonary hypertension and right heart failure in these patients; but considering the fact that the primary pathology is the physical obstruction of pulmonary vasculature, there is no surprise that their effects are only transient at best. Right ventricular failure may show some improvement with combination of diuretics and vasodilators, but because the failure is due to a mechanical obstruction it will not resolve until the obstruction is removed. Similarly, the prognosis is unaffected by medical therapy [12, 13], which should be regarded as only supportive. Because of the bronchial circulation, pulmonary embolization seldom results in tissue necrosis. Surgical endarterectomy therefore will allow distal pulmonary tissue to be used once more in gas exchange.
Currently there is only one FDA approved drug for the treatment of CTEPH in patients deemed to have inoperable disease and also patients who continue to have residual pulmonary hypertension following pulmonary thromboendarterectomy. Riociguat is a new drug in the class of soluble guanylate cyclase stimulators that has been shown to increase 6 min walk distance and decrease PVR in patients with CTEPH [20]. The observed improvements in exercise capacity and PVR of the patients in this study continue to remain significantly inferior to surgery and their durability is not proven. Pulmonary thromboendarterectomy remains the gold standard treatment and determination of inoperable disease should be made by an experienced center. Initiation of medical treatment in a patient with potentially operable disease may prevent someone from a curative procedure [21], or result in delay of referral.
Chronic anticoagulation represents the mainstay of the medical regimen. Anticoagulation is primarily used to prevent future embolic episodes, but it also serves to limit the development of thrombus in regions of low flow within the pulmonary vasculature. Inferior vena caval filters are used routinely to prevent recurrent embolization.
Operative Procedure
Pulmonary thromboendarterectomy is a technically demanding operation that is performed only in select centers around the world. Proper patient selection, meticulous surgical technique, and vigilant postoperative management have contributed to the success of this operation. A true endarterectomy (not an embolectomy) of all affected parts of the lung is essential to clear all affected areas of the pulmonary vasculature. It is clear that pulmonary endarterectomy relieves pulmonary hypertension by improving lung ventilation-perfusion match, improving right ventricular function and tricuspid regurgitation, limiting retrograde extension of clot obstruction, and preventing arteriopathic changes in the remaining patent small pulmonary vessels [14, 15]. Furthermore with resolving pulmonary hypertension, the right ventricle will regress to a normal size and improve its overall function.
The description of a surgical procedure for removal of thromboembolic material dates back to 1908, when Trendelenburg [16] first illustrated an approach in a dying patient. However it was not until the introduction and development of cardiopulmonary bypass when more procedures with better outcomes were performed. By the mid 1980s there were a total of 85 reported cases that were managed surgically but still carried a high mortality rate of about 22 % [17]. Although there have been other reports of surgical treatment of CTEPH, most of the surgical experience in pulmonary endarterectomy has been reported from the UCSD Medical Center [7, 11], and it is this experience that forms the basis of this chapter.
With our growing experience now accounting for over 3300 of these procedures, we know that there are certain principles of this procedure that have to be adhered to. Although an endarterectomy is possible even if one deviates from these principles, a successful and complete endarterectomy is not, and such outcomes are questionable. What follows is a description of the techniques of this procedure highlighting the fundamental points.
Technical Principles of the Procedure
There are several guiding principles for this operation. First and foremost the approach must be bilateral; because, for pulmonary hypertension to be a major factor, both pulmonary arteries must be substantially involved. Furthermore, it is extremely unlikely to have unilateral disease as the result of thromboembolism. In fact we believe that a small subgroup of our patients who truly do have unilateral disease, perhaps suffer from an underlying pulmonary vascular pathology with subsequent thrombosis, rather than true thromboembolism. The only reasonable approach to both pulmonary arteries is through a median sternotomy incision. Historically, there have been many reports of unilateral operation, and occasionally this is still performed with various results in inexperienced centers, through a thoracotomy. However, the unilateral approach ignores the disease on the contralateral side, subjects the patient to hemodynamic jeopardy during the clamping of the pulmonary artery, and does not allow good visibility because of the continued presence of bronchial blood flow. In addition, collateral channels develop in chronic thrombotic hypertension not only through the bronchial arteries but also from diaphragmatic, intercostal, and pleural vessels. The dissection of the lung in the pleural space via a thoracotomy incision can therefore be extremely bloody. The median sternotomy incision, apart from providing bilateral access, avoids entry into the pleural cavities, and allows the ready institution of cardiopulmonary bypass.
Cardiopulmonary bypass is an essential part of this operation and integral to ensure cardiovascular stability during the procedure. In addition cardiopulmonary bypass allows cooling the patient in preparation of circulatory arrest. Given the extent and the location of thromboembolic material, which have now transformed into a scar like fibrotic tissue adherent to the pulmonary vasculature, superior visibility is required. This is only achievable in a bloodless field so the surgeon can define an adequate endarterectomy plane and then can follow the pulmonary endarterectomy specimen deep into the subsegmental vessels. Because of the copious bronchial blood flow usually present in these patients, periods of circulatory arrest are necessary to ensure perfect visibility. Again, there continue to be sporadic reports of performing this operation without circulatory arrest with various outcomes. However, it should be emphasized that although endarterectomy is clearly possible without circulatory arrest, a complete endarterectomy is not. Surgeons claiming success with a complete endarterectomy without circulatory arrest are likely to leave behind distal disease in the subsegmental branches without ever recognizing it. We always initiate the procedure without circulatory arrest, and depending on the collateral flow through the bronchial arteries and other channels, a variable amount of dissection is possible before the circulation has to be stopped, but never a complete dissection. The circulatory arrest periods are typically limited to 20 min, with restoration of flow between each arrest. With experience, a complete endarterectomy usually can be performed within a single period of circulatory arrest on each side.
The next principle of this operation relies mainly on the experience of the operator in recognizing the true endarterectomy plane of the media, and following the specimen to its feathered tail end in each branch. It is essential to appreciate that the removal of visible thrombus is largely incidental to this operation. Indeed, in most patients, no free thrombus is present; and on initial direct examination, the pulmonary vascular bed may appear normal. The early literature on this procedure indicates that thrombectomy was often performed without endarterectomy, and in these cases the pulmonary artery pressures did not improve, often with the resultant death of the patient.
Surgical Technique
The surgical approach for this procedure is through a median sternotomy to gain access to both sides. Typically the right heart is severely enlarged, with a tense right atrium and a variable degree of tricuspid regurgitation. There is usually significant right ventricular hypertrophy and associated right heart failure, and with critical degrees of obstruction, the patient’s condition may become unstable with the manipulation of the heart. Care must be taken to avoid any unnecessary manipulation of the heart while the patient is safely placed on cardiopulmonary bypass.
Anticoagulation is achieved with the use of heparin sodium (400 units/kg, intravenously) administered to prolong the activated clotting time beyond 400 s. Full cardiopulmonary bypass is instituted with high ascending aortic cannulation and bi-caval cannulation. The heart is emptied on bypass, and a temporary pulmonary artery vent is placed in the midline of the main pulmonary artery about 1 cm distal to the pulmonary valve. The insertion site can then be used for the beginning of the left pulmonary arteriotomy. The patient is then actively cooled to a core temperature of about 18–20 °C.
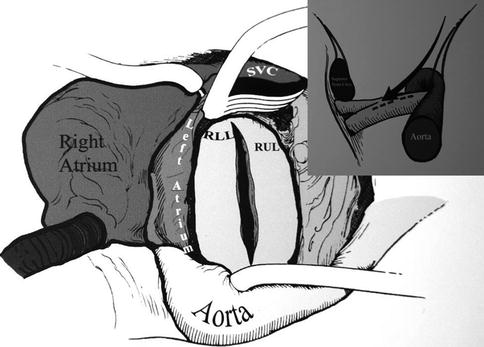
Initially, it is most convenient for the primary surgeon to stand on the patient’s left side, and perform the endarterectomy on the right side. The superior vena cava is also fully mobilized. The approach to the right pulmonary artery is made medial, not lateral, to the superior vena cava. Once the superior vena cava is fully mobilized, and the core temperature has reached 20 °C, an aortic cross clamp is applied and myocardial protection is provided through a single dose of antegrade cold blood cardioplegia (1 L). The entire procedure is now performed with a single aortic cross-clamp period with no further administration of cardioplegic solution. Additional myocardial protection is provided by using a cooling jacket surrounding the heart throughout the remainder of the procedure. Both tourniquets are now secured around the superior and inferior vena cavae to ensure complete drainage and to avoid any air entry in the venous cannulae during circulatory arrest.
A modified cerebellar retractor is then used to expose the pulmonary artery between the aorta and the superior vena cavae. An incision is made in the right pulmonary artery from beneath the ascending aorta out under the superior vena cava and entering the lower lobe branch of the pulmonary artery just after the take-off of the middle lobe artery (Fig. 15.3). It is important that the incision stays in the center of the vessel and continues in the middle of the descending pulmonary artery into the lower, rather than the middle lobe artery. The incision is carried past the take-off of the middle lobe artery.