Other Pleural Diseases
PATHOGENESIS OF PLEURAL FIBROSIS
Most of the diseases discussed in this chapter deal with pleural fibrosis. It is therefore appropriate to start this chapter with a brief discussion of the pathogenesis of pleural fibrosis. It should be noted that the process that leads to a pleurodesis after the injection of a sclerosing agent is probably very similar to that which leads to fibrosis. The reader is referred to Chapter 4 and Chapter 10 for discussions on the mechanisms of pleurodesis.
The initial step in the production of pleural fibrosis is almost always an inflammatory response in the pleura, whether it is in response to infection, an immunological process, asbestos, or other inflammatory processes. Subsequent interactions among resident and inflammatory cells, cytokines, growth factors, and blood-derived products are important in determining whether the inflammatory process will resolve or fibrosis will result. The balance between the procoagulant system and the fibrinolytic system is important in determining the outcome of an inflammatory insult to the pleura. If the procoagulant system dominates, then pleural fibrosis will develop whereas, if the fibrinolytic system dominates, no pleural fibrosis will result (1). Another important factor in the production of pleural fibrosis is angiogenesis. If vascular endothelial growth factor (VEGF) inhibitors are administered after the pleura is injured, the fibrosis is markedly reduced (2). The reader is referred to review articles (1,3) for a more in-depth discussion of the pathogenesis of pleural fibrosis.
PLEURAL DISEASE DUE TO ASBESTOS EXPOSURE
Exposure to asbestos can cause several different types of pleural disease (4). First, it can lead to a diffuse malignant mesothelioma, as described in Chapter 11; second, it can lead to a benign pleural effusion, as described in Chapter 23; third, it can bring about the development of pleural plaques or calcification; fourth, it can lead to massive pleural fibrosis; and fifth, it can produce a localized pleural abnormality called rounded atelectasis, which is easily confused with a parenchymal tumor. Pleural plaques, massive pleural fibrosis, and rounded atelectasis are discussed in this chapter.
PLEURAL PLAQUES
These hyalinized fibrous tissue collections are located predominantly in the parietal pleura at the lateral and posterior intercostal spaces; over the mediastinal pleura, particularly at the pericardium; and over the dome of the diaphragm. These locations generally correspond to areas involved in the clearance of particles from the pleural space by the lymphatics (5). Pleural plaques are one of the earliest and most common manifestations of asbestos exposure and can serve as a marker for clinically relevant asbestos exposure. Although malignant transformation has never been demonstrated in a pleural plaque, the presence of large plaques (>4 cm) has been associated with an increased risk of developing mesothelioma (5). The large plaques serve as an indication of heavy exposure, but it is believed that these plaques do not undergo a malignant transformation.
Prevalence
The prevalence of pleural plaques is somewhat dependent on the population studied. Hillerdal (6) reviewed the chest radiographs of a sizable proportion of the residents of Uppsala, Sweden, and found that the prevalence of pleural plaques in those individuals older than 40 had increased from 0.2% in 1965 to 2.7% in 1985.
The prevalence of pleural plaques was 22% in 91 elevator construction workers who probably had been exposed to low levels of asbestos in their work (7). The incidence of pleural plaques at autopsy has varied from 0.5% to 58% (8,9). When 16 separate studies with a total of 7,085 routine autopsies were combined, the prevalence of pleural plaques was 12.2% (8). The standard chest radiograph identifies between 50% and 80% of the pleural plaques that are actually present (8).
The prevalence of pleural plaques was 22% in 91 elevator construction workers who probably had been exposed to low levels of asbestos in their work (7). The incidence of pleural plaques at autopsy has varied from 0.5% to 58% (8,9). When 16 separate studies with a total of 7,085 routine autopsies were combined, the prevalence of pleural plaques was 12.2% (8). The standard chest radiograph identifies between 50% and 80% of the pleural plaques that are actually present (8).
Pleural plaques slowly develop in patients exposed to asbestos. Epler et al. (10) reviewed the chest radiographs of 1,135 patients who had been exposed to asbestos and reported that none of the patients developed pleural plaques during the 10 years after the initial exposure, and the incidence was still only approximately 10% 20 years after the initial exposure (10). Forty years after the initial exposure, however, more than 50% of the patients had radiologically visible pleural plaques. The mean duration between the initial exposure to asbestos and the development of pleural plaques was 33 years in the series of Hillerdal (6). These plaques usually calcify within several years of becoming evident radiologically. Calcification of the pleural plaques rarely occurs within the first 20 years of initial exposure to asbestos, but by 40 years, more than one third of these individuals have calcified pleural plaques (10).
Pleural plaques can also develop in individuals who are not occupationally exposed to asbestos. Kilburn et al. (11) reported that the prevalence of pleural abnormalities was 5.4% in the chest radiographs of 280 wives of asbestos workers who were initially exposed to asbestos at least 20 years previously. Churg and DePaoli (12) reported four cases of pleural plaques found at autopsy in individuals who resided in or near the chrysotile mining town of Thetford Mines, Quebec, but who did not work with asbestos. Mineral analysis of the lungs revealed that the individuals with pleural plaques had higher levels of tremolite but comparable levels of chrysotile than did the lungs of nine control subjects without pleural plaques. Constantopoulos et al. (13) reported that the prevalence of pleural calcification was 47% in 688 inhabitants of the Metsovo area in northwest Greece, an area where a solution containing tremolite was used to whitewash the houses.
Pathogenesis
Convincing evidence links pleural plaques to previous asbestos exposure. Kiviluoto (14) reviewed the place of residence of all individuals with bilateral pleural calcification in Finland and demonstrated that almost all such subjects lived near open asbestos pits. Hillerdal (6) reported that 88% of 1,596 adults older than 40 with pleural plaques had an occupational exposure to asbestos. Many patients who have pleural plaques at autopsy have a work history in which asbestos exposure would be expected (15,16). Most pleural plaques contain many submicroscopic asbestos fibers that can be demonstrated by transmission electron microscopic examination, selective area electron diffraction, and microchemical analysis of particles (17,18). In a large study (19) from France, 5,545 patients with a history of asbestos exposure had a HRCT and 882 (16%) had pleural plaques. Patients who had pleural plaque had a longer time since their first exposure and a greater cumulative exposure.
Ferruginous bodies (asbestos bodies), long considered the histologic hallmark of exposure to asbestos (20), consist of fibers coated by complexes of hemosiderin and glycoproteins and are believed to be formed by macrophages that have phagocytized the particles. Although these bodies have been shown to form from foreign inorganic and organic fibers of many different types, ferruginous bodies in most human lungs have asbestos as a core and are commonly known as asbestos bodies (20). Patients with pleural plaques have higher numbers of asbestos bodies in their lungs than do patients without pleural plaques (15,21,22). Similarly, the higher the number of asbestos bodies in the lungs, the more likely the presence of pleural plaques (20,22). It should be noted, however, that the number of asbestos fibers that are uncoated or bare (and visible only on electron microscopy) exceeds the number of asbestos bodies, which are visible by light microscopy, by 5- to 10,000-fold (23).
It appears that the various types of asbestos fibers differ in their ability to induce pleural plaques. Exposure to crocidolite is most frequently associated with the production of pleural plaques. In North America, pleural plaques are more likely to result from tremolite than from chrysotile exposure. Churg et al. (24) correlated the presence of pleural plaques with the fiber type, fiber concentration, and fiber size as determined by analytic electron microscopy in 94 longterm chrysotile miners. They found that patients with pleural plaques had a significantly higher length-width ratio for the tremolite fibers than did those without plaques (24). It is believed by some that tremolite rather than chrysotile is responsible for pleural plaques in the asbestos miners in Canada (25).
Not all pleural plaques are due to asbestos exposure (26). Zeolite minerals are aluminum silicates that
are widespread in the earth’s crust. Erionite is a zeolite that is found in old volcanic sites such as in Turkey, New Zealand, areas of Japan, and in southwestern United States. Erionite is also present in gravel pits in North Dakota, and pleural plaques were present in 3 of 15 gravel pit or road maintenance workers (20%) in one study (27). In a few villages in Turkey, the mineral has been used in buildings and for road construction, and a large percentage of the population has fiber-related pleural changes (28). One case of diffuse pleural thickening has been attributed to this fiber in Nevada (29). Wollastonite, a silicate that can be fibrous and is used in ceramics, has been reported to cause pleural plaques (30). Talc, another mineral that is a flaky silicate, has been reported to be associated with plaque formation, but this mineral is often contaminated with amphiboles, so the relationship remains to be proved (31). Pleural plaques, which may or may not be calcified, occur in other pneumoconioses including those caused by mica, Bakelite, calcimine, tin, barite, silica, and kaolin (26). They also occur after exposure to manmade vitreous fibers (26). However, concomitant exposure to asbestos is sometimes responsible for the pleural plaques seen with such diseases (17).
are widespread in the earth’s crust. Erionite is a zeolite that is found in old volcanic sites such as in Turkey, New Zealand, areas of Japan, and in southwestern United States. Erionite is also present in gravel pits in North Dakota, and pleural plaques were present in 3 of 15 gravel pit or road maintenance workers (20%) in one study (27). In a few villages in Turkey, the mineral has been used in buildings and for road construction, and a large percentage of the population has fiber-related pleural changes (28). One case of diffuse pleural thickening has been attributed to this fiber in Nevada (29). Wollastonite, a silicate that can be fibrous and is used in ceramics, has been reported to cause pleural plaques (30). Talc, another mineral that is a flaky silicate, has been reported to be associated with plaque formation, but this mineral is often contaminated with amphiboles, so the relationship remains to be proved (31). Pleural plaques, which may or may not be calcified, occur in other pneumoconioses including those caused by mica, Bakelite, calcimine, tin, barite, silica, and kaolin (26). They also occur after exposure to manmade vitreous fibers (26). However, concomitant exposure to asbestos is sometimes responsible for the pleural plaques seen with such diseases (17).
The mechanism by which asbestos fibers produce pleural plaques is unknown. Kiviluoto (14) proposed that pleural plaques are formed in response to inflammation of the parietal pleura. When an asbestos fiber is inhaled, it passes toward the periphery of the lung. Kiviluoto (14) suggested that the fiber pierces the visceral pleura and then rubs against and irritates the parietal pleura during respiratory movements. The resulting parietal pleural inflammation then gradually evolves into the hyaline plaque, which eventually calcifies. If this theory were correct, however, one would expect to find adhesions between the visceral and parietal pleura in the areas of pleural plaques, as well as long asbestos fibers in the parietal pleura.
Hillerdal (32) has suggested that the short submicroscopic fibers are primarily responsible for the pleural plaques because these fibers can be demonstrated in the plaques. He proposes that these short fibers reach the pleural space by penetrating the pulmonary parenchyma and the visceral pleura. These fibers are then removed from the pleural space, as is all particulate matter, by the lymphatic vessels that lie in the parietal pleura. Some fibers are caught in the lymphatic vessels, however, and the presence of the fiber, in conjunction with the appropriate inflammatory cell, causes pleural plaques to form over many years. One observation that does not support this hypothesis is the following: the location of black spots in the parietal pleura that represent areas where organic and inorganic material are sequestered in the pleural lymphatics do not correspond to locations where pleural plaques are found (33). A third hypothesis for the pathogenesis of pleural plaques is that the microfibrils embolize to the parietal pleura by either the parenchymal lymphatic plexus or through the costal vascular supply. Then once present in the parietal pleura, the fiber itself or agents carried by the fiber appear to be responsible for initiating and promoting the inflammatory response. Bernstein et al. (34) have demonstrated that in rats the inhalation of amosite asbestos for 5 days results in fibers penetrating the visceral pleural wall and within the parietal pleura within 7 days with a concomitant inflammatory response by 14 days. In contrast, no inflammatory response was found when chrysotile asbestos was inhaled (34).
If asbestos is injected intratracheally, it migrates to the pleura. In one study in rats, the asbestos fibers appeared in the pleural space within 3 days of intratracheal injection (35). Over a 30-day period, there were two peaks in the appearance of the asbestos fibers in the pleural space. The first peak occurred on day 7, at which time the mean length of the fiber was 1.2 µm. The second peak occurred on day 21 when the mean length of the fiber was only 0.3 µm (35).
The intrapleural injection of either crocidolite or chrysotile asbestos fibers leads to the development of a pleural effusion (36,37). Sahn and Antony (37) injected chrysotile asbestos fibers into normal rabbits, which developed exudative pleural effusions within 4 hours. Over the next 120 hours, there was increasing metabolic activity in the pleural fluid, as evidenced by a falling pH and an increasing Pco2. The animals developed pleural plaques that were evident by 7 days and developed completely by 1 month. Interestingly, if the rabbits were made neutropenic, they still developed the pleural effusion but subsequently developed marked pleural fibrosis and did not develop pleural plaques. The neutropenic rabbits did not have a macrophage influx as did the normal rabbits. These workers concluded that the pleural macrophage is important in localizing the asbestos fiber and in the ultimate formation of the pleural plaque. When a critical number of macrophages is not present, disorganization and widespread fibrosis occur (37).
Several studies have demonstrated that the exposure of mesothelial cells in cell culture to asbestos particles can induce the cells to produce substances associated with the development of fibrosis. If rat pleural mesothelial cells are exposed to crocidolite or chrysotile
asbestos fibers, the asbestos fibers are actively phagocytized and incorporated within the phagosomes. Both types of asbestos also stimulate the mesothelial cells to produce fibronectin, a substance with fibroblast chemoattractant activity (38). In contrast, quartz and carbonyl iron particles do not induce similar changes (38). After interaction with cells, asbestos fibers can also trigger a number of signaling cascades involving mitogen-activated protein kinases and nuclear factor κ-B (39). These signaling cascades can result in the production of various inflammatory mediators such as tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), IL-6, IL-8, and transforming growth factor β (TGF-β) (39). The induction of TGF-β is particularly noteworthy because it is one of the most fibrogenic agents ever discovered. Indeed, the intrapleural injection of TGF-β produces dense pleural fibrosis (40).
asbestos fibers, the asbestos fibers are actively phagocytized and incorporated within the phagosomes. Both types of asbestos also stimulate the mesothelial cells to produce fibronectin, a substance with fibroblast chemoattractant activity (38). In contrast, quartz and carbonyl iron particles do not induce similar changes (38). After interaction with cells, asbestos fibers can also trigger a number of signaling cascades involving mitogen-activated protein kinases and nuclear factor κ-B (39). These signaling cascades can result in the production of various inflammatory mediators such as tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), IL-6, IL-8, and transforming growth factor β (TGF-β) (39). The induction of TGF-β is particularly noteworthy because it is one of the most fibrogenic agents ever discovered. Indeed, the intrapleural injection of TGF-β produces dense pleural fibrosis (40).
Pathologic Features
Macroscopically, pleural plaques appear as discrete, raised, irregularly shaped areas separated by normal or slightly thickened pleura (21). These plaques are always present on the parietal pleura and found most commonly on the posterior wall of the lower half of the pleural space. Pleural plaques on the costal pleura usually have an elliptical shape, running parallel to the ribs superiorly and inferiorly (21). Pleural plaques usually do not occur in the apices of the pleural cavities or in the costophrenic angles (21). The thinner plaques are only slightly raised above the pleural surface and are grayish white in color, whereas the thicker plaques are ivory or cream colored. The diameter of the plaques varies from a few millimeters to 10 cm (21). The pleural plaques are usually multiple, and the costal pleura can look like an archipelago of different-sized plaques (31). At thoracoscopy the striking smooth whiteness of the plaques has been likened to the appearance of icing on a cake (41).
Microscopically, the plaques consist of collagenous connective tissue containing few cells (18,21). The connective tissue is arranged in a coarse, basketweave pattern and contains only a few capillaries. Normal mesothelium covers the plaques. The boundary between a plaque and the surrounding normal pleura is always sharply demarcated (21). Elastin stains show the continuity of the lamellae beneath the plaque with the surrounding normal parietal pleural connective tissue. Some calcium deposition is present in a high proportion of plaques (21). Although no asbestos fibers are visible by light microscopy, electron microscopic study demonstrates many submicroscopic fibers in almost all plaques (18).
Radiologic Features
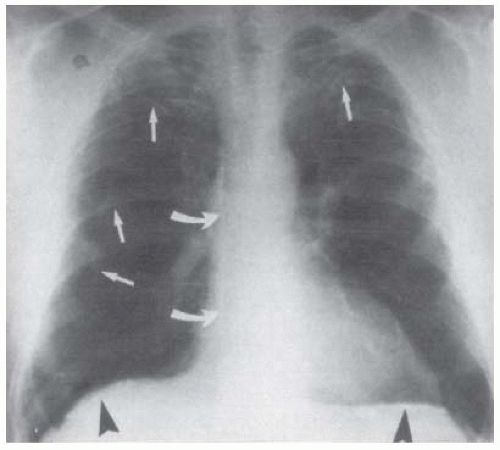
Noncalcified pleural plaques are frequently not visible on the posteroanterior (PA) and lateral chest radiographs (15,16). The earliest radiologically visible change is a line of increased density adjacent to a rib (Fig. 27.1), usually the seventh or the eighth (17,18,32). As the plaque enlarges, it becomes elliptical and protuberant, with tapering superior and inferior margins typical of an extrapleural lesion. A plaque rarely extends vertically for more than four interspaces. The thickness of the plaque varies from 1 to more than 10 mm but is usually in the range of 1 to 5 mm. Involvement of the apices or the costophrenic angles by pleural plaques is rare. Pleural plaques are usually bilateral and are often symmetric. When the pleural plaques are unilateral, they are left sided approximately 75% of the time (42). In addition, if the disease is bilateral, there tends to be more disease on the left side (43). However, one recent study was unable to demonstrate a greater amount of plaque on the left. Gallego (44) recently performed computed tomography (CT) on 40 adults with asbestos exposure and reported that the average total plaque area on the right of 47.8 cm2 was not significantly different from the average plaque area on the left of 45.3 cm2.
On a standard chest radiograph, pleural plaques are most clearly defined when viewed tangentially, that is, in profile along their long axes. A routine PA
chest radiograph distinctly demonstrates a plaque located on the inner surface of the lateral chest wall because the x-ray beam passes through more of the plaque. Noncalcified pleural plaques are best visualized by radiography at 110 to 140 kV, whereas calcification within plaques is best demonstrated at 80 kV. When the x-ray beam is perpendicular to the plaque, the plaque is presented in a frontal or en face orientation. When viewed en face, small, noncalcified plaques are difficult to see and are perceived as illdefined, irregular densities adjacent to the ribs. The en face plaque rarely appears uniformly rounded; rather, it shows a peripheral irregularity of contour that has been likened to the fringe of a map or a lily leaf (17). Because of its faintness in outline, the plaque is often overlooked or is dismissed as an artifact.
chest radiograph distinctly demonstrates a plaque located on the inner surface of the lateral chest wall because the x-ray beam passes through more of the plaque. Noncalcified pleural plaques are best visualized by radiography at 110 to 140 kV, whereas calcification within plaques is best demonstrated at 80 kV. When the x-ray beam is perpendicular to the plaque, the plaque is presented in a frontal or en face orientation. When viewed en face, small, noncalcified plaques are difficult to see and are perceived as illdefined, irregular densities adjacent to the ribs. The en face plaque rarely appears uniformly rounded; rather, it shows a peripheral irregularity of contour that has been likened to the fringe of a map or a lily leaf (17). Because of its faintness in outline, the plaque is often overlooked or is dismissed as an artifact.
Conventional and high-resolution computed tomography (HRCT) scans are more sensitive at detecting pleural plaques than is the standard chest radiograph. In one study of 159 asbestos-exposed workers with a normal chest radiograph, pleural plaques were detected in 59 (37.1%) by CT scan. The conventional CT detected pleural plaques in 58 of the patients, whereas the HRCT detected the pleural plaques in only 48 cases (45). On CT, plaques appear as discrete soft tissue or calcified thickening of the pleural surface (Fig. 27.2). Focal plaques are commonly observed in the posterior and paraspinous regions of the thorax, areas that are poorly seen on chest radiographs.
 FIGURE 27.2 ▪ Computed tomography scan of the patient in Fig. 27.1 after administration of intravenous contrast material shows numerous bilateral calcified pleural plaques (arrows), characteristic of asbestos-related pleural disease. |
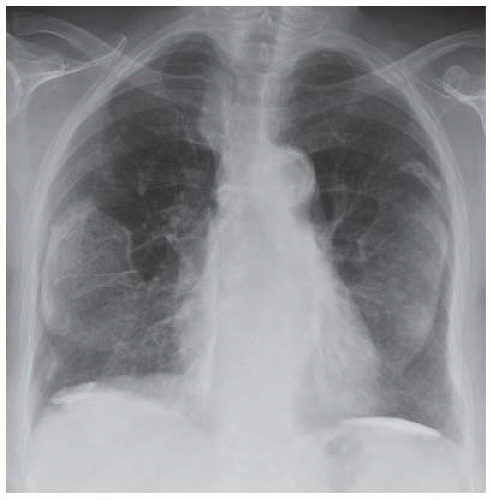 FIGURE 27.3 ▪ “Soft tissue” image from dualenergy digital subtraction chest radiography of a man with a prior history of asbestos exposure demonstrating virtual absence of ribs and thickening of the pleura. (Courtesy of Dr. Robert C. Gilkeson.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|