(1)
Department of Neuroscience, University of Turin Ospedale Molinette, Turin, Italy
Abstract
Nonatherosclerotic vasculopathies comprise a great variety of diseases, as listed in Table 17.1, that result from different etiologies; they include collagenopathies, immunological, hematological, and infection mechanisms, and other rarer causes that have in common the possibility of affecting the cerebral vessels, leading to ischemia and sometimes hemorrhage. Indeed cerebral arteries can be involved in many systemic angiitis or other generalized vasculopathies and can be selectively affected in some diseases such as the primary angiitis of the central nervous system (PACS), the Moyamoya disease, and the reversible cerebral vasoconstriction syndrome. In the majority of these diseases, the angiogram shows such findings as occlusion and irregular narrowing, alternating with dilation and sometimes aneurysm. These changes are not typical of one specific disease, but are common to all, independent of their basic pathology. Furthermore, in many cases where the involved vessel is in the precapillary–capillary sector, the angiogram can be normal, so the final diagnosis is made based on clinical laboratory findings and neuroradiological examinations of the brain parenchyma including CT and MR. In some cases brain biopsy is the essential final step. It is beyond the scope of this book to treat each of these pathologies since there is already a very rich literature about it. We report here only a few more specific studies (Hayreh 1981; Tan et al. 1982; Bogousslavsky et al. 1985; Collado et al. 1989; Beattie et al. 1995; Singleton et al. 1995; Govoni et al. 2001; Hoffman et al. 2002; Weygand and Goronzy 2003; Lucivero et al. 2004). Some angiographic examples are presented in Figs. 17.1, 17.2, and 17.3.
17.1 Great Variety of Diseases
Nonatherosclerotic vasculopathies comprise a great variety of diseases, as listed in Table 17.1, that result from different etiologies; they include collagenopathies, immunological, hematological, and infection mechanisms, and other rarer causes that have in common the possibility of affecting the cerebral vessels, leading to ischemia and sometimes hemorrhage. Indeed cerebral arteries can be involved in many systemic angiitis or other generalized vasculopathies and can be selectively affected in some diseases such as the primary angiitis of the central nervous system (PACS), the Moyamoya disease, and the reversible cerebral vasoconstriction syndrome. In the majority of these diseases, the angiogram shows such findings as occlusion and irregular narrowing, alternating with dilation and sometimes aneurysm. These changes are not typical of one specific disease, but are common to all, independent of their basic pathology. Furthermore, in many cases where the involved vessel is in the precapillary–capillary sector, the angiogram can be normal, so the final diagnosis is made based on clinical laboratory findings and neuroradiological examinations of the brain parenchyma including CT and MR. In some cases brain biopsy is the essential final step. It is beyond the scope of this book to treat each of these pathologies since there is already a very rich literature about it. We report here only a few more specific studies (Hayreh 1981; Tan et al. 1982; Bogousslavsky et al. 1985; Collado et al. 1989; Beattie et al. 1995; Singleton et al. 1995; Govoni et al. 2001; Hoffman et al. 2002; Weygand and Goronzy 2003; Lucivero et al. 2004). Some angiographic examples are presented in Figs. 17.1, 17.2, and 17.3.
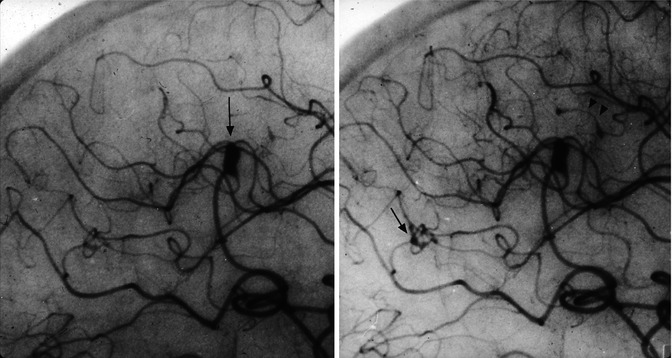
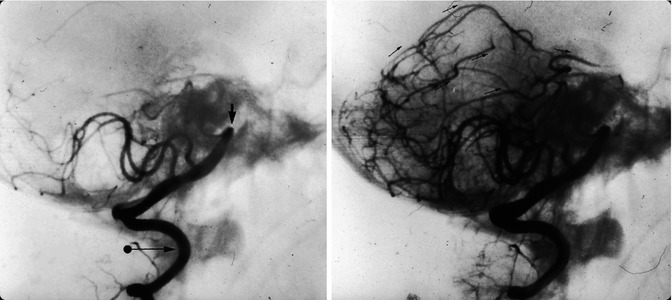
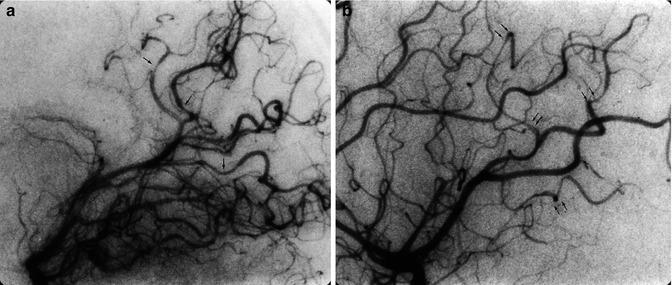
Table 17.1
Nonatherosclerotic vasculopathies
Primary angiitis of the CNS |
Granulomatous vasculitis, affecting predominantly the precapillary arterioles, occasionally large vessels (such as ICA, PCA, MCA, ACA). Small aneurysms have also been reported. Angiography frequently normal |
Systemic vasculitis that can affect the CNS |
Giant cell arteritis: granulomatous vasculitis involving predominantly superficial temporal ophthalmic arteries, occasionally extracranial ICA and VA |
Takayasu arteritis: granulomatous vasculitis |
Polyarteritis nodosa: necrotizing vasculitis; large intracranial vessels can be involved; aneurysm may be present |
Wegener’s granulomatosis: necrotizing vasculitis; can involve intracranial arteries with ischemia due to occlusion and hemorrhage due to rupture of arteries |
Behçet’s disease: no signs of necrotizing or granulomatous vasculitis are found; frequently perivenular infiltrations are present; angiography often normal |
Systemic diseases that can affect the CNS |
Systemic lupus erythematosus |
Rheumatic disease |
Sjogren’s syndrome |
Behçet’s disease: no signs of necrotizing or granulomatous vasculitis are found; angiography frequently normal |
Crohn’s disease |
Sarcoidosis |
Hematological conditions |
Sickle cell disease, Moschcowitz’s disease, platelet hyperaggregability, disseminated intravascular coagulation, antiphospholipid syndrome, deficiency of factors involved in coagulation (deficiency of proteins C and S, antithrombin III, factor V Leyden), homocystinuria |
Vasculopathies not linked to inflammatory processes |
Neurofibromatosis, intracranial vessel occlusion, possible intracranial aneurysm |
Ehlers-Danlos syndrome (aneurysm, frequently dissecting, of the extra- and intracranial arteries; dissecting aneurysm of the intracavernous ICA with spontaneous cavernous fistulas) |
Fibromuscular dysplasia |
Scleroderma |
Livedo reticularis (Sneddon’s syndrome) |
Moyamoya disease |
Infection diseases |
Bacterial, fungal, parasitic, viral (Varicella- zoster; herpes simplex; citomegalovirus; HIV ) |
Others |
Vasculopathies related to drugs, radiotherapy, intracranial tumors, trauma |
Migraine stroke |

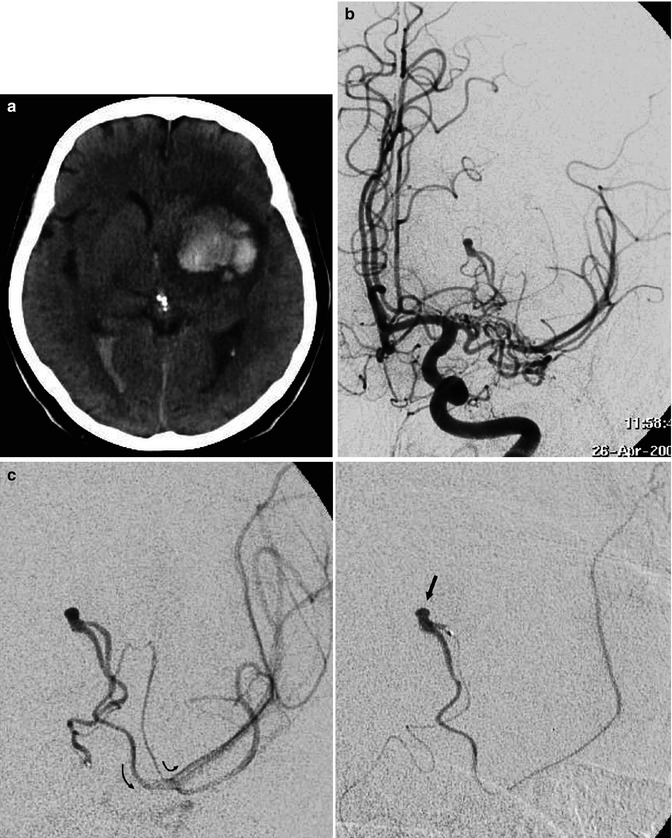
Fig. 17.1
Lateral carotid angiogram (detail with magnification) in a patient with polyarteritis nodosa, early and later phases. Aneurysmal dilatations (arrow) and stenotic changes (arrowheads) are present

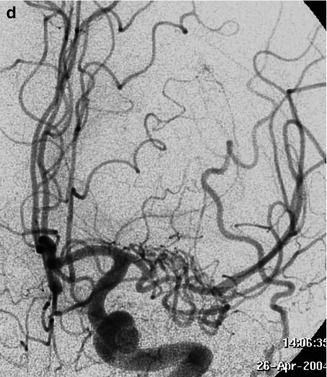
Fig. 17.2
A young boy with neurofibromatosis presenting with an acute occlusion of the basilar artery. Vertebral angiogram showing the occlusion (arrow). In the later phases, retrograde injection of the superior cerebellar artery (SCA) and anterior inferior cerebellar artery (AICA) through opening of the anastomosis with the posterior inferior cerebellar artery (PICA; arrows). There is a slight displacement of the vertebral artery at C1–C2 owing to dysplasia of the cervical spine (arrow with dot)

Fig. 17.3
Suspected primary angiitis in a young boy presenting with sudden onset of epileptic seizures and psychic impairment. Lateral carotid (a) and vertebral angiogram (b). Irregularity and focal narrowing of several distal branches in the anterior and posterior circulation are visible
A few of these diseases will be described more in detail due to their clinical relevance and particular clinicopathological aspects.
17.2 Cerebrovascular Fibromuscular Dysplasia
Fibromuscular dysplasia (FMD) is a systemic vascular disease, characterized by nonatherosclerotic changes involving the wall of small- to medium-sized arteries. All vascular territories can be affected; among them, FMD is seen in the renal arteries most frequently, and this is often responsible for hypertension in these patients. Palubinskas and Ripley (1964) and Palubinskas and Newton (1965) were the first to report the angiographic aspects of this disease in the cephalic arteries. Numerous reports followed (Palubinskas et al. 1966; Andersen 1970; Wylie et al. 1966; Houser and Baker 1968; Bradac and Heymat 1970; Stanley et al. 1974; Manelfe et al. 1974; Osborn and Anderson 1977; Bradac and Oberson 1983), clearly distinguishing FMD from atherosclerotic and other nonatherosclerotic lesions and its possible relationship with stroke.
The true incidence of FMD is unknown. In some reports, it is cited as up to 10 % in angiographic studies (Chiras et al. 1985). FMD is more frequent in women, and it is rare in children (Andersen 1970).
17.2.1 Pathology and Etiopathogenesis
FMD is commonly characterized by involvement of the medial layer, with fibrous proliferation and hyperplasia of the smooth muscle cells. Less commonly, FMD involves the intima or adventitia. These changes lead to thickening and fibrosis of the layer involved. The precise etiopathogenesis has yet to be identified. A deficiency of alpha 1-antitrypsin has been reported (Schievink et al. 1998).
17.2.2 Diagnosis
The aspect of the arteries involved can be typical and is characterized on the angiogram, CT angiography, or MRI angiography by multifocal stenosis, which is sometimes severe, alternating with areas of mural dilatation, leading to the so-called string-of-beads appearance. Pseudoaneurysms and tubular stenosis can occur.
In the latter situation, in a patient with stroke, the differential diagnosis of dissection can sometimes be difficult. On control angiograms, the findings remain unchanged in FMD, while in dissection normalization frequently occurs. The vessel most often involved is the extracranial internal carotid artery (ICA) in its midportion, frequently bilaterally (Osborn and Anderson 1977), followed by the extracranial vertebral artery (VA) in its mid-distal portion. Multiple vessels are commonly affected (Osborn and Anderson 1977; Osborn 1999). Intracranial vessels may also be affected, but this occurs less frequently (Elias 1971; Frens et al. 1974; Tomasello et al. 1976; Saygi et al. 1990). Examples are represented in Fig. 17.4a–e.
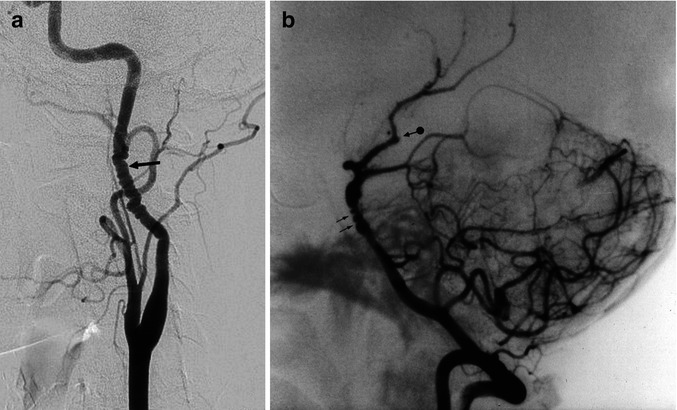
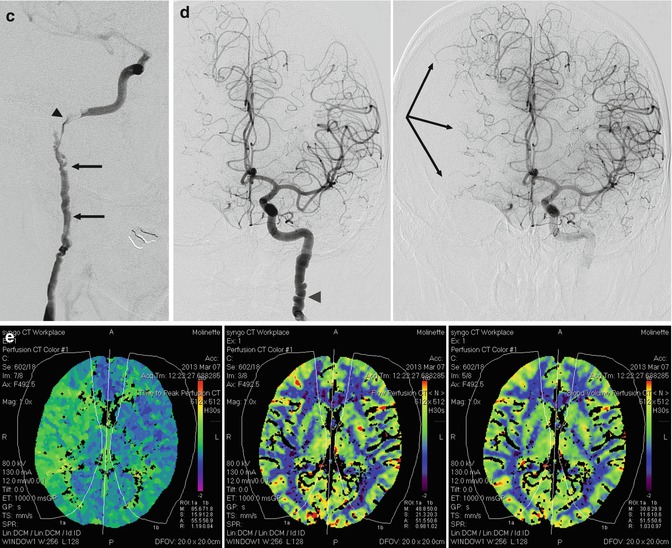


Fig. 17.4
(a) Typical string-of-beads appearance of the internal carotid artery (arrow). (b) String-of-beads appearance at the level of the distal basilar artery (arrows) associated with a similar finding involving the posterior cerebral artery (PCA) and SCA, with occlusion, probably embolic, of the distal branches (arrow with dot). (c–e) Middle-aged woman presenting with one episode of right amaurosis fugax. On the admission in the hospital, she was clinically normal. The CT angiography followed by a complete conventional angiographic study revealed a severe fibromuscular dysplasia with dissection of the right extracranial ICA. (c) Right ICA angiogram. Typical string-of-beads appearance (arrows). Dissection close to the skull base where probably an intraluminal thrombus is present (arrow head). There is a slowdown of the intracranial circulation. The A1 is not recognizable. (d) Left ICA angiogram (early and later phases) showing similar changing of the extracranial ICA (arrow head). There is aplasia of the right A1. Pial anastomoses from right ACA toward MCA (arrows). Pial anastomoses from PCA toward MCA were visible on the vertebral angiogram. There was aplasia of P1. A rich collateral circulation occurred through the right ECA (see Fig. 3.12d). (e) CT perfusion study showed, on the image on the left, a slight delay of the time to peak on the right hemisphere, but no changes of the CBF and CBV were demonstrated on the two images on the right. The patient underwent medical therapy
FMD is frequently associated with cerebral aneurysm (Handa et al. 1970b; Hirsch and Roessmann 1975; Osborn and Anderson 1977; Paulson et al. 1978). Carotid-cavernous sinus and vertebral artery with perivertebral veins fistulas have also been reported (Geraud et al. 1973; Bradac et al. 1985; Hieshima et al. 1986).
17.2.3 Clinical Relevance
The extracranial changes of FMD can occasionally be found in patients examined for other pathologies. It can, however, be the cause of ischemic stroke owing to the formation of thrombus at the site of the stenosis, which is followed by intracranial embolization. The close relationship of dissection with FMD is well established. Intracranial FMD can be the cause of ischemia or be responsible for dissecting aneurysms (Küker et al. 2011) with possible subarachnoid hemorrhage (SAH). Finally, there is a frequent association with intracranial berry aneurysms, which are responsible for the SAH in these patients. The treatment (medical, surgical, endovascular) depends on the type of lesion and stroke.
17.3 Moyamoya Disease
This is a chronic, progressive cerebrovascular disease, first described in Japanese patients by Takeuchi and Shimizu in 1957. Other Japanese reports followed (Kudo 1968; Nishimoto and Takeuchi 1968). The term Moyamoya, proposed by Suzuki and Takaku (1969), means “vague puff of smoke.” Despite the fact that the disease is more frequent in Japanese patients, later studies showed that it can occur also in non-Asians (Taveras 1969; Galligioni et al. 1971; Picard et al. 1974a, b; Huber 1979; Bradac and Oberson 1983; Masuda et al. 1993). Children and young patients are prevalently involved, but the disease may also be seen in older patients.
17.3.1 Pathology and Etiopathogenesis
Intimal thickening due to proliferation and migration of smooth muscle cells leads to progressive occlusion of the intracranial distal ICA, with extension also to the M1 and A1. A rich network of vessels develops, involving perforators of the middle cerebral artery (MCA), anterior cerebral artery (ACA), posterior communicating artery, P1, and the anterior and posterior choroidal arteries. The significance of this network is not completely clear. Some authors (Taveras 1969; Crouzet et al. 1974; Picard et al. 1974a, b) have suggested that this is a kind of collateral circulation, with anastomoses between the deep and superficial vascular territories. This interpretation is intriguing since deep perforators are commonly thought to be end arteries, with no links to one another or to the medullary arteries. However, microangiographic studies (Kodama and Suzuki 1974; Umansky et al. 1985) (see also Sect. 15.7.1) have shown that a few anastomoses can be present among the single perforators and between the perforators and medullary arteries. It is therefore conceivable that in slowly occurring occlusive diseases like Moyamoya, anastomoses occur in these vascular territories (Fig. 17.5).