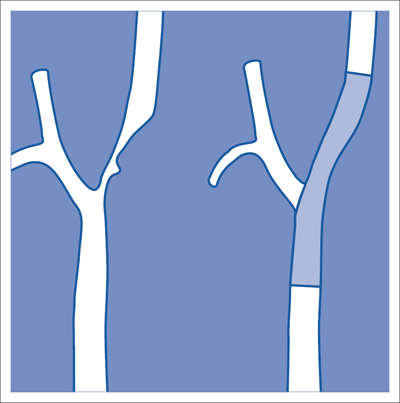
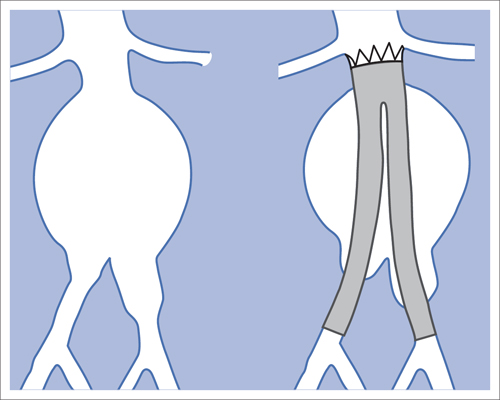
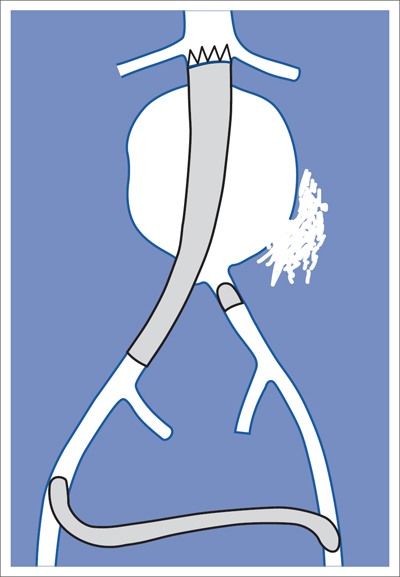
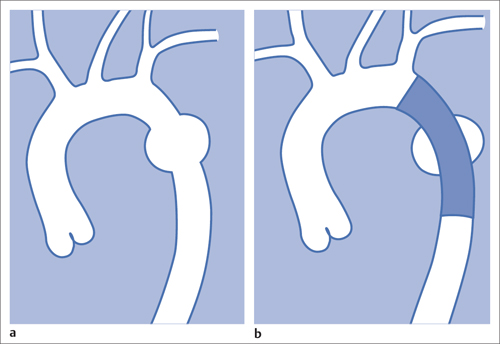
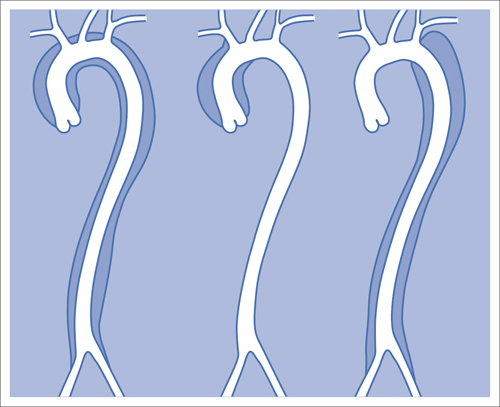
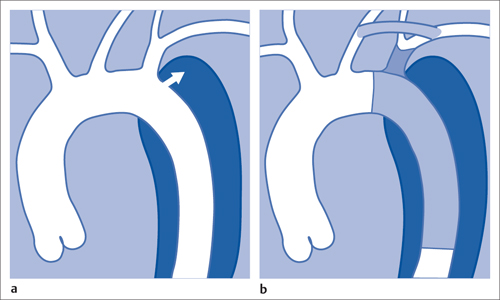
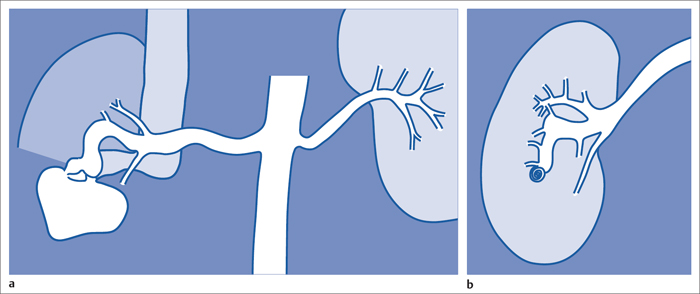
7 Other Endovascular Interventions The methods presented here are either performed only in special facilities or they require extensive experience or a specific clinical environment. They are described briefly to provide a comprehensive overview of the capabilities of endovascular interventions. However, this discussion is neither intended nor sufficient to serve as a practical guide for performing these procedures. Note: Neuroradiologic and cardiological interventions are not discussed here. Stent placement in carotid artery stenoses has been the subject of more discussion in recent years than any other vascular intervention. This has not been because of technical difficulty but because of the specific risks and because its indication is defined in competition with established surgical therapy. As elastic arteries with a low rate of restenosis (5 to 8%), the carotid arteries provide a favorable setting for endovascular treatment. The crucial problem lies in the risk of acute complications from embolism or spasm. Balloon dilation was initially the predominant procedure (Mathias 1981). Today self-expanding stents are normally used. In the majority of cases they are placed across the origin of the external carotid artery. Some are therefore supplied in a specially adapted design. Following angiography a sheath is introduced into the common carotid artery over a stiff glide wire (the end of the wire lies in the external carotid artery). Next the internal carotid artery is catheterized. Preliminary dilation is performed in applicable cases, and then the stent is placed (Fig. 7.1). A protection system in place of the 0.014 in. guidewire can provide a track for introducing the balloon catheter and stent. This system consists of a wire with an umbrella-like or saclike filter that is expanded cranial to the region being treated. Its purpose is to capture any plaque or thrombus material from the stenotic region that may be mobilized during treatment. The comparative studies available to date suggest that stent placement is not inferior to surgical treatment in terms of quality of results and complications. Particularly in this sensitive region, thorough and conscientious deliberation is crucial in determining the indication. Equally crucial is good cooperation with vascular surgeons and neurologists. The surgical treatment of aortic aneurysms essentially consists of placing a synthetic tube or a Y-shaped bifurcation prosthesis in the aneurysmal sac. The tube or prosthesis is then joined to the nondilated connecting vessels by proximal and distal sutures. Similar synthetic prostheses can be advanced into the aneurysmal sac through sheaths of sufficient width and length via the femoral arteries, obviating the need to open the abdomen (Fig. 7.2). Elastic wire constructions incorporated into the prosthesis create a tight connection to the abdominal aorta proximal to the aneurysm and to the iliac arteries distal to it. Hooks attached to the outer circumference are pressed into the aortic wall with a large balloon. The prosthesis with its two “legs” can only be introduced from one side. This means that the limb for the contralateral side must be pulled down from the contralateral side and usually lengthened. The width of the prosthesis body must be precisely measured and ordered beforehand. The length of each limb is often adapted to the individual anatomy only during the procedure itself. In some cases one must forgo this type of treatment because certain anatomical conditions are not met. The important thing is that there are no leaks at the junctions between the prosthesis and the vessels and also at the joints between the sections of the prosthesis. Otherwise the blood pressure would continue to act against the wall of the aneurysm and the risk of a rupture would not be eliminated. An equally dangerous risk is posed by vessels arising from the aneurysm that communicate with the arterial system outside the aneurysm via other vessels such as the inferior mesenteric or lumbar arteries. Such vessels can be occluded beforehand by embolization. Or one may attempt to occlude them later if the computed tomographic (CT) scan demonstrates inflow of contrast into the treated aneurysm. The monoiliac prosthesis is an interesting option. Its simplicity makes it well suited for treating a ruptured aneurysm (Fig. 7.3). It can quickly bridge the aneurysm (eliminating the need to open the abdominal cavity). A suprapubic transverse bypass is then created to reestablish the connection to the contralateral side. The procedure also requires closing the contralateral common iliac artery with an occluder. Aneurysms and dissections of the thoracic aorta are less common than in the abdominal aorta. However, they are more likely to rupture. Moreover, open surgical treatment has a high mortality rate (10 to 30%, Ohki and Malas 2005). Implantation of endovascular prostheses for aneurysms of the descending aorta (Fig. 7.4) and for Stanford type B dissections (DeBakey 3, Figs. 7.5 and 7.6) is a viable alternative. Patients tolerate the procedure far better, and it is associated with significantly lesser mortality. Fig. 7.4 Aneurysm of the descending thoracic aorta. a Initial findings. b Treatment with an endovascular prosthesis. Fig. 7.5 Types of aortic dissection. Left: DeBakey 1, Stanford A; middle: DeBakey 2, Stanford A; right: DeBakey 3, Stanford B. Fig. 7.6 Treatment of a type B (DeBakey 3) aortic dissection with an endovascular prosthesis. a Initial findings: Dissection begins directly after the origin of the subclavian artery. b The prosthesis covers the origin of the left subclavian artery, requiring a bypass from the left common carotid artery to the subclavian artery. Pulmonary arteriovenous malformations appear on the chest radiograph as dense focal shadows with a characteristic pedicle in the direction of the pulmonary hilum. These malformations can become quite large and can occur singly or multiply. They can result in such a large shunt volume that they produce the clinical symptoms of a cyanotic heart defect. Treatment consists of embolizing the feeder branches of the pulmonary artery with large Gianturco coils (Cook Medical, Bloomington, IN, USA) or more simply with an Amplatzer occluder (St. Jude Medical, St. Paul, MN, USA). Given the great width that these fistulas can attain, the major risk of treatment is that a coil could pass through the fistula into the left heart and from there enter the systemic circulation. Congenital arteriovenous fistulas occasionally occur in the kidney as well. Here too the shunt volume can become high enough to cause cardiac problems and create a steal phenomenon that impairs the blood supply to the renal parenchyma (Fig. 7.7a). The fistula can be occluded by one or more coils of the appropriate size. Fig. 7.7 The patient is a 34-year-old man with an arteriovenous fistula in the right kidney with a venous aneurysm, strong contrast enhancement of the vena cava, and a steal effect in the caudal portion of the kidney. a Initial findings. b Fistula is occluded with a coil.
Carotid Stents
Endovascular Aortic Prostheses
Abdominal Aorta
Thoracic Aorta
Arteriovenous Malformations
Pulmonary Arteriovenous Malformations
Renal Arteriovenous Malformations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree