OTHER ACYANOTIC HEART DEFECTS
Introduction
Severe cyanotic congenital heart defects (CHDs) usually present in the early neonatal period because of physiologic abnormalities that they produce soon after birth. In contrast, acyanotic CHD rarely present with symptoms in the neonatal period unless they are very severe. CHD is generally classified into acyanotic and cyanotic defects, largely dependent upon whether they are cyanotic or not.1,2 The relative prevalence these CHDs are shown in Table 41.1. The acyanotic defects are further subdivided into obstructive and left-to-right shunt defects. In the previous chapters, coarctation of the aorta (CoA) and patent ductus arteriosus (PDA) in the premature were discussed. In this chapter, a selected group of acyanotic CHD will be reviewed briefly.
Table 41.1. Classification and prevalence of CHDs
| Type of defects | % incidence | % incidence |
|---|---|---|
| Acyanotic | 65 | |
| Obstructive lesions Pulmonic stenosis (PS) Aortic stenosis (AS) Coarctation of the aorta (CoA) | 25 10.0 7 8 | |
| Left-to-right shunt lesions Atrial septal defect (ASD) Ventricular septal defect (VSD) Patent ductus arteriosus (PDA) Atrioventricular septal defect | 40–50 10.0 25 10 5 | |
| Cyanotic Tetralogy of Fallot (TOF) Transposition of the great arteries (TGA) Tricuspid atresia Total anomalous pulmonary venous connection (TAPVC) Truncus arteriosus (TA) | 10 5 1.5 1 1 | 20 |
| Other (miscellaneous) | 15 |
Obstructive Acyanotic Congenital Heart Lesions
Among the obstructive lesions, pulmonary stenosis (PS), aortic stenosis (AS) and CoA are most common; CoA was reviewed in Chapter 38. The other 2 lesions will be presented.
Pulmonary stenosis (PS)
In PS, the narrowing may be at the valvar, subvalvar, or supravalvar level or in the branch pulmonary arteries (PAs). Valvar stenosis is the most common and constitutes 7.5% to 9.0% of all CHDs.
Valvar PS Pathologically there is fusion of the thickened pulmonary valve (PV) leaflets forming what is described as “dome-shaped” PV. Right ventricular hypertrophy, proportional to the degree of obstruction and poststenotic dilatation of main pulmonary artery (MPA), unrelated to the degree of PS are seen.3
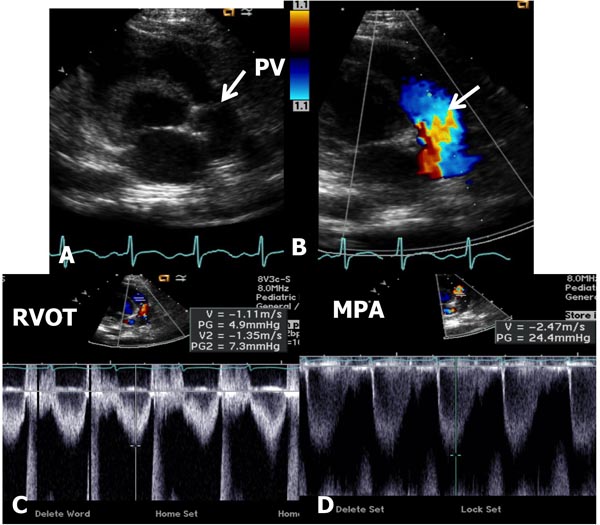
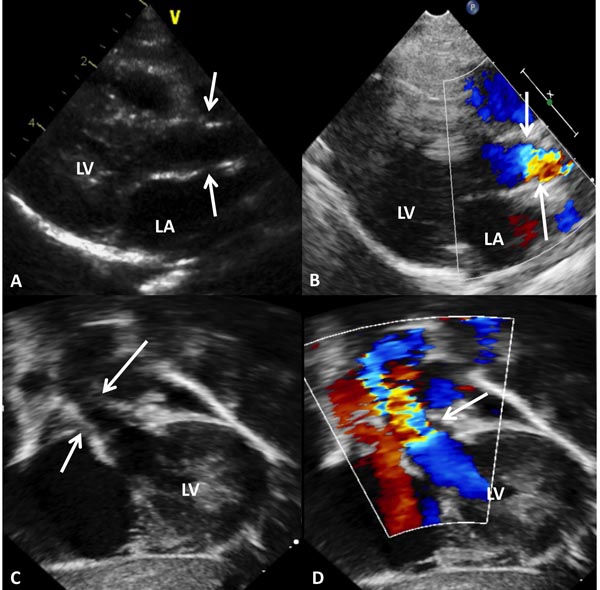
In mild-to-moderate PS, the disorder may be undetected during the neonatal period or a cardiac murmur may be noticed on routine examination. Physical examination may be normal with the exception of an ejection systolic murmur at the left upper sternal border. Chest x-ray shows normal-sized heart with dilatation of the MPA segment. The ECG may show normal neonatal right ventricular preponderance or may manifest right ventricular hypertrophy. Echocardiogram may show thickened and domed PV (Figure 41.1A) with turbulent flow (TF) by color Doppler imaging (Figure 41.1B) and increased Doppler flow velocity by pulsed and continuous wave Doppler (Figure 41.1C). The latter may be used to calculate gradient across the PV using modified Bernoulli equation (gradient = 4V2, where V is the peak velocity across the PV in meter/second). It should be warned that such gradient calculation may underestimate the degree of obstruction in the first few days of life because of normally high PA pressures.
Figure 41.1. Selected video frames from precordial short-axis views of neonate with an ejection systolic murmur heard best at the left upper sternal border demonstrating somewhat domed PV (arrow) in A with flow acceleration at the PV level (arrow) in B. Pulse Doppler velocity in the right ventricular outflow tract (RVOT) was normal at 1.1 m/s (C) with an increase in the velocity in the MPA to 2.4 m/s. The estimated gradient was 24 mmHg (see insert in D) suggesting mild PS. Sometimes, the Doppler data may underestimate the gradient because of normally high PA pressures in the very early neonatal period.
For the babies with mild-to-moderate PS, no treatment is necessary, but they should be reevaluated periodically.
Other types of right ventricular obstructions such as infundibular PS, double-chamber right ventricle (RV), supravalvar PA stenosis (with or without Williams syndrome) and discrete sub-pulmonary membrane in the right-sided morphologic left ventricle (LV) of corrected transposition patients may also occur,3 but are relatively rare in the neonate and will not be reviewed.
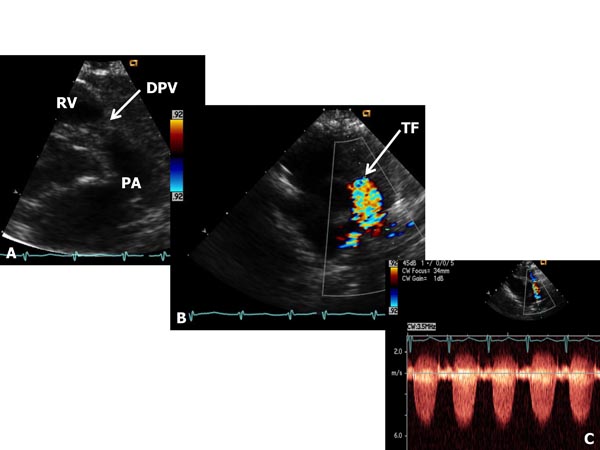
Critical PS The term critical PS is generally reserved to describe very severe PV obstruction producing suprasystemic right ventricular systolic pressure, right-to-left shunt across the atrial septum and/or ductal-dependent pulmonary blood flow (PBF). These babies most usually present with cyanosis and/or a cardiac murmur. Physical examination demonstrates cyanosis and a long ejection systolic murmur at the left upper sternal border. Increased jugular venous pulsations and hepatomegaly may be seen in some babies. In some infants holosystolic murmur of tricuspid insufficiency may be heard at the left lower sternal border. Chest x-ray may show cardiomegaly and decreased pulmonary vascular markings. ECG shows right ventricular hypertrophy. Echocardiogram shows severe right ventricular hypertrophy and markedly increased gradient across the PV (Figure 41.2). Right-to-left shunt across the patent foramen ovale (PFO) and left-to-right shunt across the PDA may be demonstrated by color flow imaging. At first, prostaglandin E1 (PGE1) infusion should be started to open the ductus and to provide PBF. This should be followed by percutaneous balloon pulmonary valvuloplasty (see Chapter 20). If the procedure could not be performed because of technical reasons or balloon valvuloplasty was unsuccessful in relieving the obstruction, surgical pulmonary valvotomy may be required. In some infants additional aortopulmonary shunt (modified Blalock–Taussig [BT] shunt) may be needed, especially if there is hypoplasia of the RV. In babies with less severe obstruction balloon valvuloplasty may be performed later, past the neonatal period. For more detailed discussion, the reader is referred elsewhere.4–7
Figure 41.2. Selected video frames from parasternal short-axis views of neonate with a long and loud ejection systolic murmur heard best at the left upper sternal border, demonstrating somewhat domed and dysplastic pulmonary valve (DPV) (arrow) in A with TF originating at the PV level (arrow) in B. Continuous wave Doppler velocity across the valve level in C shows a marked increase (in excess of 5.5 m/s) suggesting severe (critical) PS.
Branch PA stenosis In utero, the majority of the right ventricular output is directed toward the Ao via the ductus arteriosus (DA).8,9 Consequently, the lungs receive only 7% of combined ventricular output (CVO) (see Chapter 1). Therefore, the branch PAs are small in size. At birth, the pulmonary vascular resistance (PVR) falls, resulting in increased pulmonary flow (50% of CVO). To accommodate this increased blood flow, the velocity of flow in the branch PAs has to increase causing turbulence across these vessels. Such flow dynamics result in producing a cardiac murmur. With time, the branch PAs enlarge and the physiologic branch PS resolves.
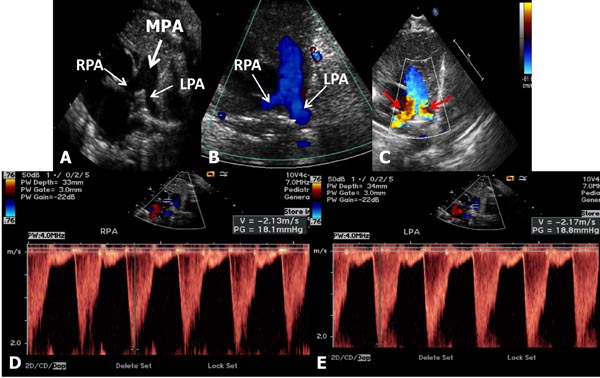
Babies with branch PS usually present with a murmur that is heard in the left upper sternal border and axillae, or it may be detected on echo-Doppler studies performed for some other reason. They are otherwise asymptomatic and usually have no other positive cardiac findings. Chest x-ray and ECG are normal. Echocardiogram reveals somewhat small branch PAs (Figure 41.3) with increased Doppler flow velocity across the branch PAs (Figure 41.3D and Figure 41.3E). No treatment is necessary and the murmur disappears within 6 to 12 weeks and if an echocardiogram is repeated, the above described findings normalize.
Figure 41.3. Selected video frames from precordial short-axis views of 2 neonates to image the MPA, left pulmonary arteries (LPA) and right pulmonary arteries (RPA). Normal 2D appearance in A with laminar flow in B suggests normal PAs. In another neonate with ejection systolic murmurs in the axillae and back, there is TF (red arrows in C) with increased Doppler flow velocity (>2.1 m/s) in the RPA (D) and LPA (E) suggesting peripheral PA stenosis. This is probably physiological and likely to resolve with time.
Aortic Stenosis
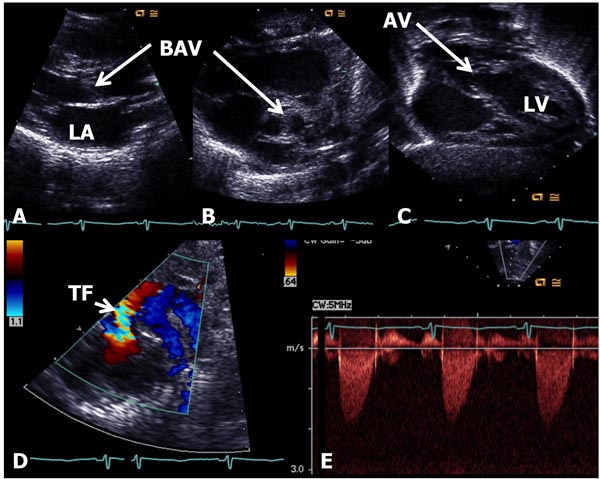
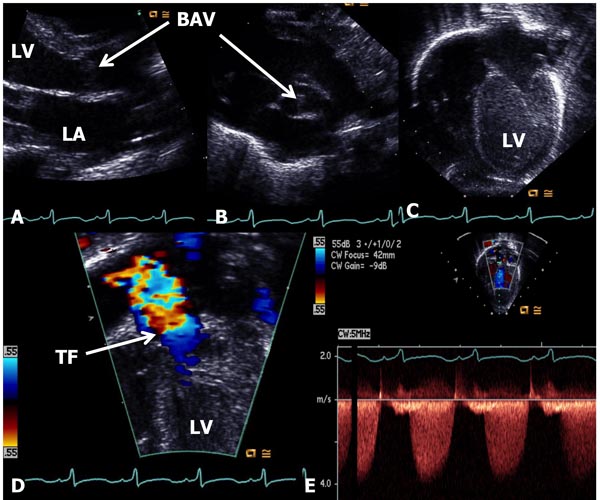
In AS, the obstruction may occur at valvar, subvalvar (fixed subaortic stenosis and idiopathic hypertrophic subaortic stenosis), and supravalvar sites.10 Valvar stenosis is the most common, and the prevalence of congenital valvar AS is 5% to 6% of patients with CHD. Most common pathology in AS is bicuspid valve, although tricuspid and rarely unicuspid aortic valve (AV) leaflets may also occur. There is varying degrees of commissural fusion causing AV obstruction. Dysplastic AV leaflets with or without hypoplasia of the valve ring may also be seen, particularly in neonates and young infants. The valve leaflets are thickened, domed and nonpliable. Concentric hypertrophy of the left ventricular muscle is present and is largely proportional to the degree of narrowing. Poststenotic dilatation of the ascending aorta (AAo) is seen in most cases, and the degree of such dilatation is independent of the severity of AS. In mild-to-moderate AS, a cardiac murmur may be heard on routine examination or the infant may be undetected during the neonatal period. Physical examination may be normal with the exception of an ejection systolic click at the apex and an ejection systolic murmur at the right upper sternal border. Chest x-ray shows normal-sized heart. The ECG is usually normal. Echocardiogram may show bicuspid aortic valve (BAV) (Figure 41.4B); the valve leaflets may be thickened (Figure 41.4A) and domed (Figure 41.4C) with TF by color Doppler imaging (Figure 41.4D). The Doppler flow velocity across the AV is increased (Figure 41.4E). The latter maybe used to calculate gradient across the AV using modified Bernoulli equation (gradient = 4V2, where V is the peak velocity across the AV in meter/second). Mean Doppler gradients may more accurately represent true peak-to-peak gradient.11
Figure 41.4. Selected video frames from echocardiographic studies of a neonate with an ejection systolic murmur heard best at the right upper sternal border demonstrating thickened AV (A) and BAV (B) with doming of the AV (C). Color Doppler image shows TF at the AV (arrow) in D with low (<2 m/s) Doppler velocity (E) across the AV, indicating very mild AS with BAV. AV, aortic valve; BAV, bicuspid AV; TF, turbulent flow; LA, left atrium; LV, left ventricle.
No treatment is necessary for babies with mild-to-moderate AS, but should be followed periodically to ensure detection of worsening degree of obstruction.
Fixed subaortic membranous stenosis (Figure 41.5), idiopathic hypertrophic subaortic stenosis (hypertrophic cardiomyopathy) (Figure 41.6) and supravalvar AS (Figure 41.7), the latter associated with Williams syndrome, may also be seen in the neonate but are less common than valvar AS and will not be discussed further.
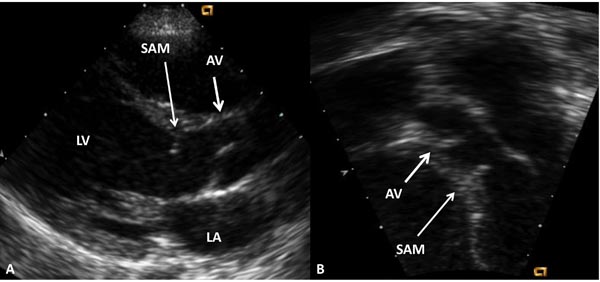
Figure 41.5. Selected video frames from parasternal long-axis (A) and apical 5-chamber (B) echocardiographic views demonstrating subaortic membrane (SAM). The AV is marked. Doppler studies (not shown) showed increase in Doppler flow velocity at the level of SAM. LA, left atrium; LV, left ventricle.
Figure 41.6. Selected video frames from parasternal long- (A) and short-axis (B) echocardiographic views demonstrating markedly thickened interventricular septum (arrows), indicative of hypertrophic cardiomyopathy. Doppler studies (not shown) showed increase in Doppler flow velocity at the level of the thickened interventricular septum. AV, aortic valve; LA, left atrium; LV, left ventricle.
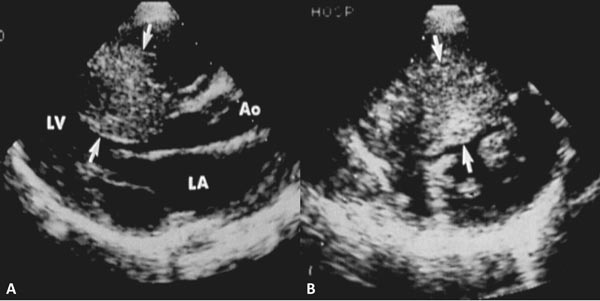
Figure 41.7. Selected video frames from parasternal long-axis (A and B) and subcostal (C and D) echocardiographic views demonstrating supravalvar AS (arrows). The narrowing is above the AV. Color Doppler showed flow turbulence (arrows in B and D) and increase in Doppler flow velocity (not shown) at the level of supravalvar AS. LA, left atrium; LV, left ventricle.
Critical AS The term critical AS is used to describe very severe AV stenosis with a high peak systolic pressure gradient across the AV, signs and symptoms of congestive heart failure (CHF), and/or ductal-dependent systemic circulation. The pressure gradient across the AV may not be high in some babies because of poor left ventricular function. Echocardiographic features of 2 neonates with severe obstruction are shown in Figures 41.8 and 41.9.
Figure 41.8. Selected video frames from echocardiographic studies of a neonate with AS demonstrating thickened AV (A) and BAV (B) with dilated LV (C). Color Doppler image shows TF at the AV (arrow) in D with higher (≈4 m/s) Doppler velocity (E) across the AV, indicating moderate to severe AS with BAV.
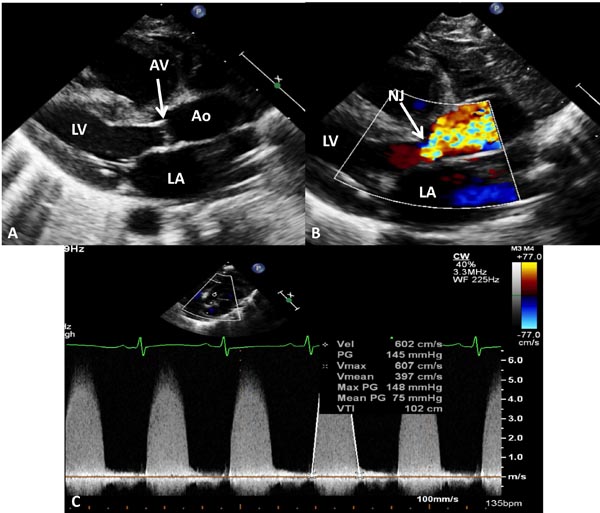
Figure 41.9. Selected video frames from echocardiographic studies of another neonate with very severe AS demonstrating thickened and domed (A) AV with color Doppler image shows TF with narrow jet (NJ) at the AV (arrow) in B with very high Doppler velocity (C) across the AV, indicating a maximum peak instantaneous gradient of 148 mmHg and a mean gradient of 75 mmHg, suggestive of very severe AS. The AV was bicuspid (not shown). AAo, ascending aorta; LA, left atrium; LV, left ventricle.
In these babies, relief of obstruction should be undertaken on an urgent basis. Balloon aortic valvuloplasty is considered an acceptable alternative to surgical aortic valvotomy in the management of critical AS in the neonate12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree