Percutaneous coronary intervention of severely calcified lesions has historically been associated with major adverse cardiac event (MACE) rates as high as 30%. In the ORBIT II (Evaluate the Safety and Efficacy of OAS in Treating Severely Calcified Coronary Lesions) trial, treatment of de novo severely calcified lesions with the Diamondback 360° Coronary Orbital Atherectomy System (OAS) resulted in low rates of procedural and 30-day adverse ischemic events. The long-term results from this trial have not been reported. We sought to determine the 1-year outcomes after orbital atherectomy of severely calcified coronary lesions. ORBIT II was a single-arm trial enrolling 443 subjects at 49 US sites with severely calcified lesions usually excluded from randomized trials. OAS utilizes a centrifugal differential sanding mechanism of action for plaque modification prior to stent implantation. After OAS drug-eluting stents were implanted in 88.2% of the patients. The primary safety end point was 30-day MACE, the composite of cardiac death, myocardial infarction, or target vessel revascularization [TVR]. The present analysis reports the 1-year follow-up results from ORBIT II. One-year data were available in 433 of 443 patients (97.7%), with median follow-up time of 16.7 months. The 1-year MACE rate was 16.4%, including cardiac death (3.0%), myocardial infarction (9.7%), and target vessel revascularization (5.9%). The 1-year target lesion revascularization rate was 4.7%, and stent thrombosis occurred in 1 patient (0.2%). Independent predictors of 1-year MACE and target vessel revascularization were diameter stenosis at baseline and the use of bare-metal stents. In patients with severely calcified lesions who underwent percutaneous coronary intervention, the use of OAS was associated with low rates of 1-year adverse ischemic events compared with historical controls. This finding has important clinical implications for the selection of optimum treatment strategies for patients with severely calcified lesions.
Despite advances in technology, percutaneous coronary intervention (PCI) of severely calcified coronary lesions remains a challenge. Contemporary studies have shown that the presence of moderate or severe coronary calcification at the target lesion is associated with a substantial increase in death, myocardial infarction (MI), stent thrombosis, and need for revascularization. Coronary calcification is frequent, with about 1/3 of the lesions treated by PCI presenting with a substantial amount of calcification angiographically. Current therapeutic options to manage calcified lesions (e.g., cutting/scoring balloons and rotational atherectomy) are associated with a high rate of restenosis and stent failure at long-term follow-up. Recently, the ORBIT II (Evaluate the Safety and Efficacy of OAS in Treating Severely Calcified Coronary Lesions) trial demonstrated favorable procedural and 30-day outcomes with the Diamondback 360° Coronary Orbital Atherectomy System (OAS) (Cardiovascular Systems, Inc., St. Paul, Minnesota) in the treatment of severely calcified lesions, resulting in its Food and Drug Administration (FDA) approval. Whether the beneficial effects of this device seen at 30 days translate into favorable 1-year outcomes is not known. We report the prespecified analysis of the 1-year outcomes from the ORBIT II pivotal trial.
Methods
The design and initial results of ORBIT II have been published previously. In brief, ORBIT II was a prospective, multicenter, nonblinded, single-arm clinical trial that enrolled patients with de novo severely calcified coronary lesions who underwent PCI. Key study inclusion criteria included: (1) target vessel reference diameter ≥2.5 and ≤4.0 mm with a stenosis ≥70% and <100% or a stenosis ≥50% and <70% with evidence of clinical ischemia through positive stress test, fractional flow reserve value ≤0.8, or intravascular ultrasound (IVUS) minimum lumen area ≤4.0 mm 2 ; (2) target lesion length ≤40 mm; and (3) imaging evidence of severe calcium at the lesion site based on the angiographic presence of radio-opacities noted without cardiac motion before contrast injection involving both sides of the arterial wall in at least 1 location, total length of calcium of ≥15 mm and extending partially into the target lesion, or presence of ≥270° of calcium at 1 cross-section through IVUS. Patients were excluded if (1) the target vessel had a stent from a previous PCI unless the stent was on a different branch than the target lesion and was implanted >30 days before with no >30% in-stent restenosis; (2) recent MI (defined as creatine kinase-MB [CK-MB] >1× upper limit of laboratory normal [ULN] within 30 days of the index procedure); and (3) evidence of current left ventricular ejection fraction ≤25%. The ORBIT II trial conformed to ethical guidelines of the Declaration of Helsinki, and study participants provided informed consent.
The coronary OAS uses a diamond-coated eccentric crown that, while rotating over an atherectomy guide wire, expands laterally through centrifugal force (up to a maximum orbit diameter for a given rotational speed) resulting in a differential sanding of coronary calcification. Two OAS configurations were used in the ORBIT II trial, pneumatic OAS and electric OAS, with crown diameter ranging from 1.25 up to 2.00 mm. Detailed description of OAS mechanism of action can be found elsewhere. The use of other adjunctive devices, such as thrombectomy, embolic protection devices, brachytherapy, or cutting balloons, was not allowed. Pre- and postprocedure dual antiplatelet therapy was recommended to conform to the American Heart Association/American College of Cardiology/Society for Cardiovascular Angiography and Interventions joint guidelines for PCI.
The primary safety end point of ORBIT II was the rate of 30-day major adverse cardiac events (MACEs) defined as the composite of cardiac death, MI, and target vessel revascularization (TVR). MI was defined as CK-MB >3x ULN with or without new pathologic Q waves. Rate of stent thrombosis was reported and defined according to the Academic Research Consortium definition. All adverse events were adjudicated by an independent clinical events committee. All patients were followed clinically during the index hospitalization, at 30 days and at 1 year.
Continuous variables are summarized as mean ± SD. Survival curves for time-to-event variables were constructed on the basis of all available follow-up data using Kaplan-Meier methods. Multivariable Cox proportional hazards regression was performed to identify independent predictors of 1-year MACE and target vessel revascularization (TVR) (2-sided α = 0.05 for significance). The multivariable model was built with candidate variables being selected if of clinical interest and/or satisfying the criterion of p <0.2 in the univariate analysis. Variables included in the final were carefully selected to avoid overfitting and included for MACE: age, previous coronary artery bypass graft surgery, estimated glomerular filtration rate, reference vessel diameter, lesion length, diameter stenosis, and use of bare-metal stents (BMS) versus drug-eluting stents (DES), and for TVR: reference vessel diameter, diameter stenosis, and use of BMS versus DES. All statistical analyses were performed using SAS, version 9.3 (SAS Institute, Cary, North Carolina).
The ORBIT II trial was funded by Cardiovascular Systems, Inc., and was designed collaboratively by the steering committee and the sponsor. The authors had unrestricted access to the study data, drafted the manuscript, made the decision to submit for publication, and guarantee the integrity and accuracy of its content.
Results
From May 25, 2010, to November 26, 2012, 443 consecutive patients with severely calcified coronary lesions from 49 US sites were enrolled in the ORBIT II trial. Among them, 432 (97.5%) were actually treated with OAS before stent placement, and 1-year data were available in 433 patients (97.7%) (median follow-up time of 16.7 months). Baseline, angiographic, and procedural characteristics of the study population are listed in Table 1 . DES were implanted in most of the lesions treated (88.2% of stents).
| Variable | N = 443 |
|---|---|
| Age (years) | 71.4 ± 9.9 |
| Men | 286/443 (64.6%) |
| Smoker (current or former) | 293/443 (66.1%) |
| History of diabetes mellitus | 160/443 (36.1%) |
| History of dyslipidemia | 407/443 (91.9%) |
| History of hypertension | 406/443 (91.6%) |
| Estimated glomerular filtration rate (mL/min/1.73 m 2 ) | 75.8 ± 26.2 |
| Prior stroke/transient ischemic attack | 39/443 (8.8%) |
| Prior myocardial infarction | 99/443 (22.3%) |
| Prior coronary artery bypass graft surgery | 65/443 (14.7%) |
| Target coronary artery | |
| Left anterior descending | 227/440 (51.6%) |
| Left circumflex | 64/440 (14.5%) |
| Left main | 10/440 (2.3%) |
| Right | 132/440 (30.0%) |
| Ramus | 7/440 (1.6%) |
| Mean lesion length (mm) | 18.9 ± 9.0 |
| Mean diameter stenosis (%) | 84.4 ± 9.0 |
| Mean reference vessel diameter (mm) | 3.1 ± 0.4 |
| American College of Cardiology/American Heart Association lesion type | |
| Type A | 0/440 (0.0%) |
| Type B1 | 114/440 (25.9%) |
| Type B2 | 197/440 (44.8%) |
| Type C | 129/440 (29.3%) |
| Subjects with stent implanted | 432/440 (98.2%) |
| Stents implanted per patient | 1.3 ± 0.6 |
| Types of stents implanted | |
| Bare metal stent | 64/542 (11.8%) |
| Drug-eluting stent | 478/542 (88.2%) |
| First-generation drug-eluting stent | 94/478 (19.7%) |
| Second-generation drug-eluting stent | 384/478 (80.3%) |
| Total procedure time (min) ∗ | 52.5 ± 29.6 |
| Total fluoroscopy time (min) | 18.2 ± 12.3 |
| Total volume of contrast used (mL) | 173.9 ± 86.4 |
| Orbital atherectomy system device used (size in mm) | |
| Pneumatic, 1.25 | 320/457 (70.0%) |
| Pneumatic, 1.50 | 33/457 (7.2%) |
| Pneumatic, 1.75 | 2/457 (0.4%) |
| Pneumatic, 2.00 | 2/457 (0.4%) |
| Electric, 1.25 | 98/457 (21.4%) |
| Electric, 1.50 | 2/457 (0.4%) |
| Orbital atherectomy system device speed(s) used (rpm) | |
| Low only (80,000) | 93/432 (21.5%) |
| Low and high (80,000/120,000) | 317/432 (73.4%) |
| High only (120,000) | 22/432 (5.1%) |
| Total orbital atherectomy system device run time (seconds) | 66.8 ± 45.6 |
| Individual orbital atherectomy system run time (seconds) | 19.5 ± 5.7 |
∗ Total procedure time defined as the time from when the first guide catheter was placed in the access site to the time the last guide catheter was removed from the access site.
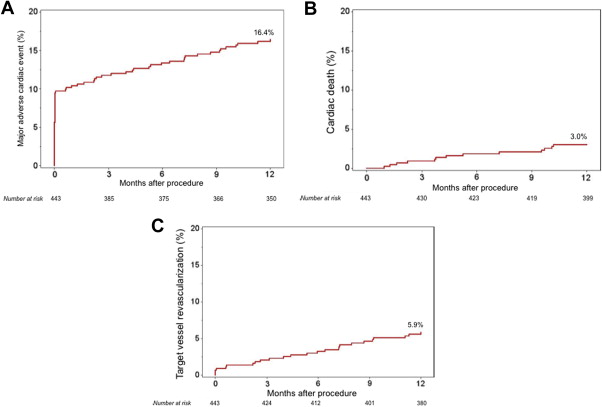
At 1 year, the primary composite end point of MACE occurred in 16.4% of patients, with cardiac death occurring in 3.0%, MI in 9.7%, and TVR in 5.9% of patients ( Figure 1 , Table 2 ). The target lesion revascularization (TLR) rate was 4.7%, and only 1 (0.2%) stent thrombosis occurred within 1 year ( Table 2 ). When using the SCAI definition of periprocedural MI (CK-MB ≥10× ULN without new Q waves or ≥5× ULN with new Q waves), the 1-year MI and MACE rates were 2.0% and 9.9%, respectively. Table 3 lists the rates of 1-year adverse clinical events stratified by stent type. Use of BMS was associated with a significantly higher rate of MACE, TVR, and TLR at 1 year.
| Variable | In-Hospital | 30-Day | 1-Year |
|---|---|---|---|
| Major adverse cardiac events | 43 (9.8) | 46 (10.4) | 72 (16.4) |
| Major adverse cardiac events (using myocardial infarction = creatine kinase-MB 10× upper limit of normal) | 11 (2.5) | 14 (3.2) | 43 (9.9) |
| Death | 2 (0.5) | 2 (0.5) | 19 (4.4) |
| Cardiac death | 1 (0.2) | 1 (0.2) | 13 (3.0) |
| Myocardial infarction (creatine kinase-MB 3× upper limit of normal) | 41 (9.3) | 43 (9.7) | 43 (9.7) |
| Non–Q-wave myocardial infarction | 38 (8.6) | 39 (8.8) | 39 (8.8) |
| Q-wave myocardial infarction | 3 (0.7) | 4 (0.9) | 4 (0.9) |
| Myocardial infarction (creatine kinase-MB 10× upper limit of normal) | 9 (2.1) | 9 (2.0) | 9 (2.0) |
| Target vessel revascularization | 3 (0.7) | 6 (1.4) | 25 (5.9) |
| Target lesion revascularization | 0 (0.0) | 3 (0.7) | 20 (4.7) |
| Target vessel revascularization non-target lesion revascularization | 3 (0.7) | 3 (0.7) | 8 (1.9) |
| Academic Research Consortium definite/probable stent thrombosis | 1 (0.2) | 1 (0.2) | 1 (0.2) |
| Variable | Bare Metal Stent (n=43) | Drug-eluting Stent (n=389) | p Value |
|---|---|---|---|
| Major adverse cardiac events | 10 (24.3%) | 56 (14.5%) | 0.047 |
| Target vessel revascularization | 6 (15.1%) | 18 (4.7%) | 0.01 |
| Target lesion revascularization | 6 (15.3%) | 13 (3.4%) | 0.002 |
| Target vessel revascularization non-target lesion revascularization | 2 (5.0%) | 6 (1.6%) | 0.15 |
Independent predictors of 1-year MACE and TVR are summarized in Table 4 . After multivariable analysis, diameter stenosis and the use of BMS emerged as independent predictors of MACE and TVR.
| Variable | Major Adverse Cardiac Events | Target Vessel Revascularization | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted Hazard Ratio (95% Confidence Interval) | p Value | Adjusted Hazard Ratio (95% Confidence Interval) | p Value | Unadjusted Hazard Ratio (95% Confidence Interval) | p Value | Adjusted Hazard Ratio (95% Confidence Interval) | p Value | |
| Age (per 10 years) | 1.02 (0.81, 1.30) | 0.84 | 0.87 (0.67, 1.12) | 0.27 | 0.99 (0.95, 1.02) | 0.45 | ||
| Diabetes mellitus | 1.00 (0.62, 1.61) | 0.99 | 1.01 (0.45, 2.29) | 0.98 | ||||
| Smoker ∗ | 0.79 (0.49, 1.27) | 0.32 | 0.64 (0.29, 1.41) | 0.27 | ||||
| Prior myocardial infarction | 1.07 (0.62, 1.84) | 0.81 | 1.10 (0.44, 2.75) | 0.84 | ||||
| Prior coronary artery bypass grafting | 1,57 (0.89, 2.78) | 0.12 | 1.21 (0.64, 2.29) | 0.56 | 1.46 (0.55, 3.88) | 0.45 | ||
| Estimated glomerular filtration rate (mL/min/1.73 m 2 ) (per 10) | 0.94 (0.86, 1.03) | 0.21 | 0.95 (0.86, 1.04) | 0.26 | 1.09 (0.95, 1.25) | 0.20 | ||
| Left ventricular ejection fraction (per 10%) | 0.94 (0.74, 1.19) | 0.61 | 1.10 (0.72, 1.69) | 0.65 | ||||
| Reference vessel diameter (mm) baseline | 0.64 (0.35, 1.16) | 0.14 | 0.74 (0.40, 1.39) | 0.35 | 0.47 (0.17, 1.33) | 0.16 | 0.37 (0.12, 1.14) | 0.08 |
| Narrowing length (mm) | 1.02 (1.00, 1.05) | 0.09 | 1.02 (0.99, 1.04) | 0.26 | 1.01 (0.97, 1.06) | 0.56 | ||
| Diameter stenosis (%) | 1.03 (1.00, 1.05) | 0.052 | 1.03 (1.00, 1.06) | 0.04 | 1.05 (1.00, 1.10) | 0.04 | 1.06 (1.00, 1.11) | 0.04 |
| Bare metal stent vs drug-eluting stent | 1.93 (1.01, 3.68) | 0.047 | 2.33 (1.17, 4.65) | 0.02 | 3.38 (1.34, 8.52) | 0.01 | 4.38 (1.69, 11.35) | 0.002 |
| 1.25 mm vs >1.25 mm crown | 1.24 (0.50, 3.08) | 0.64 | 1.02 (0.24, 4.34) | 0.98 | ||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


