2 Optimal Processing of Diagnostic Lung Specimens
Optimal specimen handling is essential for the accurate interpretation of biopsies and cytologic preparations obtained in the course of evaluating the patient with lung disease.1–9 The limited number of sampling techniques available can be divided into three general categories: bronchoscopy, transthoracic needle core biopsy or aspiration, and surgical wedge biopsy of peripheral lung through a transthoracic approach.8,10–13
The focus of this chapter is on these techniques and the specimens thereby obtained, with emphasis on how they should be prepared and handled in the laboratory. Once an appropriate sample of adequate quality has been obtained, the addition of pertinent clinical data and radiologic information greatly increases the likelihood of a meaningful and accurate diagnosis.13–15 Even when the diagnostic goal is simply to rule out malignancy, the effect of other information may be substantial, especially when the sample is of marginal quality or size. In the case of diffuse non-neoplastic lung diseases (often referred to as “interstitial lung diseases”), a reasonable amount of clinical and radiologic information is essential for accurate interpretation. Without such information, even the experienced lung pathologist may need to resort to a purely descriptive diagnosis.15
In this chapter, specimen characteristics and processing steps are presented for each of the common lung samples taken in the course of clinical evaluation for pulmonary disease. Also, for each type of sample, the benefits and limitations are reviewed. Such a working knowledge of specimen handling for each procedure ensures the greatest likelihood of success in establishing a specific diagnosis and, in the end, a rational treatment plan. An overview of biopsy procedures and the specimens generated is presented in Table 2-1.
Table 2-1 Diagnostic Sampling Techniques, Specimens Obtained, and Common Analyses Performed
| Sampling Technique | Specimens/Common Analyses |
|---|---|
| Sputum expectoration | Cytologic smears and centrifuge preparations Fixed or air-dried, then stained for cytopathologic examination Microbiologic cultures performed as indicated |
| Bronchoscopy with: Washings | Cytologic smears and centrifuge preparations Fixed or air-dried, then stained for cytopathologic examination Microbiologic cultures performed as indicated |
| Brushings | Cytologic smears and centrifuge preparations Fixed or air-dried, then stained for cytopathologic examination Microbiologic cultures performed as indicated |
| Endobronchial biopsy | Forceps tissue biopsy specimen, 2–3 mm in size Fixed and processed for histopathologic examination Microbiologic cultures and other testing performed as indicated |
| Transbronchial biopsy | Forceps tissue biopsy specimen, 2–3 mm in size Processed for histopathologic examination Microbiologic cultures and other testing performed as indicated |
| Bronchoalveolar lavage (BAL) | Cytologic smears and centrifuge preparations Fixed or air-dried, then stained for cytopathologic examination and biochemical analysis Microbiologic cultures and other testing performed as indicated |
| Transbronchial fine needle aspiration | Cytologic smears and centrifuge preparations Fixed or air-dried, then stained for cytopathologic examination Microbiologic cultures and other testing performed as indicated |
| Surgical “wedge” lung biopsy (either video-assisted or open) | 3- to 5-cm peripheral lung tissue sample including pleura and alveolar parenchyma Fixed and processed for histopathologic examination Microbiologic cultures and specialized testing performed as indicated |
| Transthoracic needle core biopsy and aspiration | Core biopsy fragment(s), cytologic smears and centrifuge preparations Smears and cellular preparations: fixed or air-dried, then stained for cytopathologic examination, with special stains for organisms and other specialized techniques as indicated Core tissue specimens: fixed and processed for histopathologic examination Microbiologic cultures and specialized assays performed as indicated |
| Thoracentesis | Cytologic centrifuge preparations Fixed or air-dried, then stained for cytopathologic examination Microbiologic cultures, biochemical analysis, and specialized assays performed as indicated |
Specimens Obtained through the Flexible Bronchoscope
The flexible bronchoscope was introduced in the United States in the late 1960s, after successful use in Japan.8 Despite several decades of experience with the rigid bronchoscope, the advent of the flexible bronchoscope (Fig. 2-1) allowed evaluation of the major conducting airways without use of general anesthesia and with less morbidity.8,16 Furthermore, the flexible instrument has the advantage of providing better access to more distal and obliquely branched airways. The rigid bronchoscope still has major uses in certain settings, mainly those in which the device’s larger bore is an advantage, but today pulmonary endoscopy is dominated by the flexible bronchoscope.
Endobronchial Biopsy
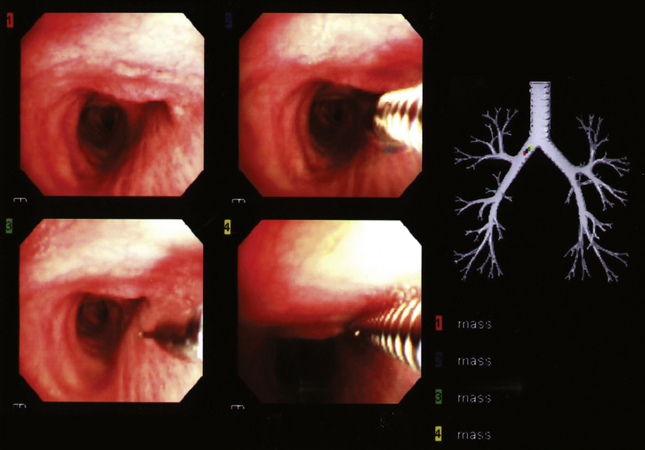
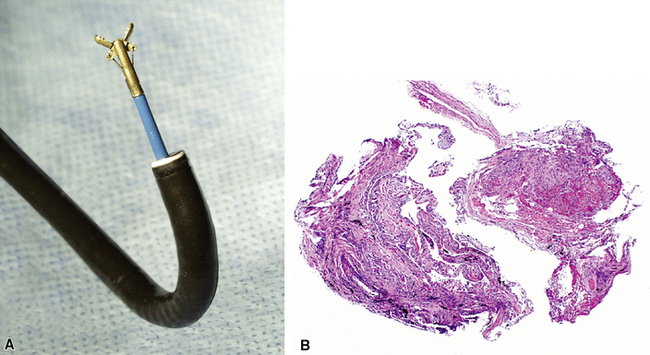
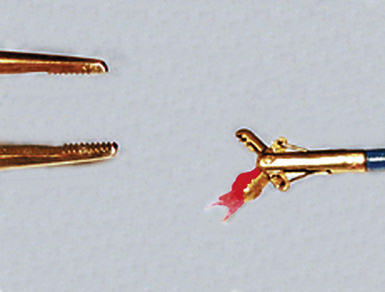
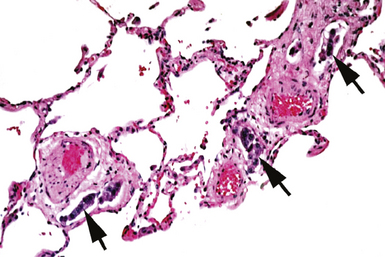
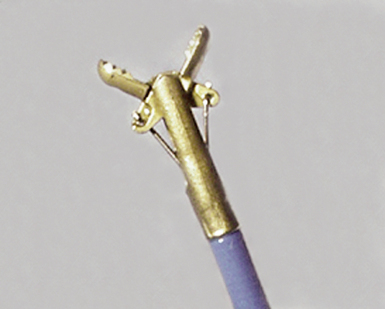
Modern flexible bronchoscopes allow the operator to accurately visualize the structural integrity of the bronchial tree and its mucosal surfaces, commonly as far distal as the sixth order bronchi17,18 (Fig. 2-2). Biopsy of visualized mucosal lesions most commonly is performed using cupped forceps (Fig. 2-3A) introduced through the flexible shaft of the bronchoscope.8 With this technique, the airway mucosa, lamina propria, and musculature are sampled with or without fragments of cartilage (see Fig. 2-3B). The closed forceps is extracted from the bronchoscope and the biopsy is dislodged from the cupped ends of the device and placed in fixative or other solution (see later discussion). A sterile needle or fine-tipped forceps (Fig. 2-4) is useful for removing the delicate tissue specimen. The tissue specimens obtained in this way average 2 to 3 mm in greatest dimension. The lymphovascular network of the peribronchial sheath often is included in these samples, making it possible to identify metastatic disease when present in lymphatic or vascular channels (Fig. 2-5).
For transferring bronchoscopic biopsy specimens from carrier or fixative solutions into cassettes for paraffin embedment, a useful device is a polystyrene pipette with the tip cut off with scissors (Fig. 2-6). This pipetting device allows the operator to transfer delicate specimens without tearing or crushing. Transferring of these specimens using forceps is to be avoided.
When infection is a consideration, bronchoscopic biopsy specimens can be transported directly to the microbiology laboratory for processing.4,19,20 In most scenarios, biopsy samples are sent for histopathologic evaluation and microbiologic studies directly from the bronchoscopy suite or bedside. For endobronchial and transbronchial samples, the optimal number of biopsy specimens varies depending on the radiologic distribution of disease,15,21–23 bronchoscopy findings,8,11,16 and the specific diagnostic entities under consideration.8,24,25 As a general guideline, if the patient is tolerating the procedure well, the greater the number of biopsy specimens, the greater the likelihood of establishing a definitive diagnosis.8,23,25
Transbronchial Biopsy
In contrast with endobronchial biopsy, the transbronchial biopsy technique is intended to sample alveolar lung parenchyma beyond the cartilaginous bronchi.8,16,17,26 This technique uses either crocodile-style (Machida) forceps or cupped forceps manipulated by the operator (Fig. 2-7). To obtain the biopsy specimen, the forceps is advanced with the jaws closed into a distal airway until resistance is met. The forceps is retracted slightly and then advanced slightly, with the jaws open. The jaws are then closed and the forceps is pulled out through the bronchoscope. Advancing the forceps at end-expiration can be helpful in forcing the bronchiolar wall and peribronchiolar lung parenchyma into the mouth of the device. The successful parenchymal biopsy specimen appears finely ragged (Fig. 2-8) and usually measures between 2 and 3 mm in diameter.26–28
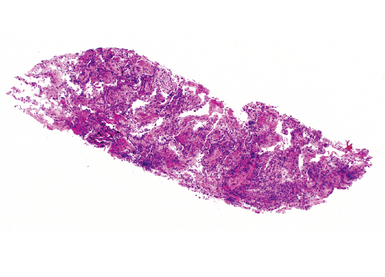
Figure 2-8 Transbronchial biopsy. Low-magnification image of a transbronchial biopsy specimen of generous size.
As with endobronchial samples, the transbronchial sample is teased from the forceps with a sterile needle, and the same precautions are advised to avoid damage during handling and transfer to fixative or other solution. The truncated pipette technique also is useful in this setting. Once processed, both types of biopsy specimens should be serially sectioned for thorough microscopic evaluation.2
Bronchial Brushings
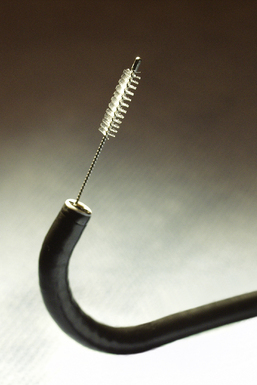
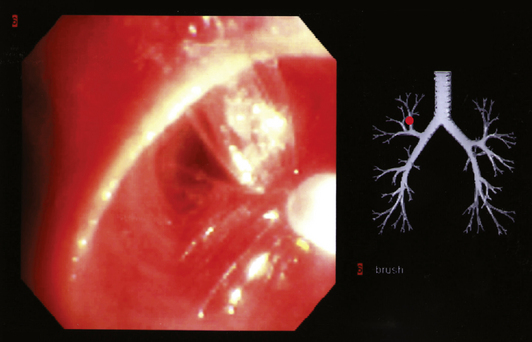
Visualized lesions of the airway epithelium can be sampled for cytologic evaluation.8,11,16 The technique involves the use of a conical bristle brush (Fig. 2-9). Under direct visualization, the brush is agitated against the mucosal surface of the airway, forcing cells into the interstices of the bristles (Fig. 2-10). The brush is removed from the bronchoscope and can be applied directly to glass slides. Cells and secretions smeared on slides can be immediately fixed for cytologic evaluation using the Papanicolaou staining method or air-dried for use with the Wright-Giemsa staining technique (this choice is best made in consultation with the pathologist). Immediate fixation of slides is best accomplished by direct immersion in 95% alcohol immediately after smearing of the sample on the slide. Each fixation and staining technique produces characteristic artifacts, and the use of one over the other depends on operator training and preference.
If slides are not available for smear preparations, the brush can be cut off and placed directly into a small vial of sterile saline, which is then shaken vigorously to dislodge cells into the fluid. This fluid sample of suspended cells is sent to the laboratory for millipore filtration or cytocentrifuge-type application onto slides,8,11,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree