Cigarette smoking is known to be deleterious to patients with coronary artery disease; however, the effect of smoking on vascular responses after coronary drug-eluting stent implantation is unknown. We sought to examine vascular response after sirolimus-eluting stent implantation in patients with ongoing smoking using optical coherence tomography, compared with former smokers and nonsmokers. We identified 181 sirolimus-eluting stents in 140 subjects who underwent follow-up optical coherence tomography imaging. Subjects were divided into 3 groups: current smokers (n = 28), former smokers (n = 35), and nonsmokers (n = 77). Stent strut coverage, neointimal characteristics, and strut malapposition were evaluated. The incidence of uncovered stent struts was significantly higher in nonsmokers compared with current smokers (13.3 ± 13.3% vs 6.7 ± 8.3%; p = 0.001). On qualitative evaluation of neointimal morphology, the prevalence of heterogeneous neointima was higher in current smokers (71.9%) than in former smokers (36.0%) or nonsmokers (10.1%) (p = 0.004 and p <0.001, respectively). There was no difference in the incidence of malapposition among the 3 groups. Multivariate modeling showed that current smoking was negatively associated with the presence of uncovered struts (odds ratio 0.33; 95% confidence interval 0.14 to 0.79; p = 0.013) and positively associated with the presence of heterogeneous neointima (odds ratio 9.47; 95% confidence interval 3.79 to 23.72; p <0.001). In conclusion, the incidence of strut coverage was higher in current smokers compared with nonsmokers. However, the pattern of neointima was more heterogeneous in current smokers.
Optical coherence tomography (OCT) has been applied to provide high-resolution in vivo images of coronary arteries and to evaluate stent status and neointimal tissue after coronary stent implantation. The aim of the present study was to examine the effect of smoking status on markers of arterial healing including strut coverage, neointimal characteristics, and malapposition assessed by OCT in patients after sirolimus-eluting stent (SES) implantation.
Methods
The Massachusetts General Hospital (MGH) OCT Registry is a multicenter registry of patients who underwent OCT of the coronary arteries and involves 20 sites across 6 countries. For the present study, we identified 146 patients with 190 SESs in de novo lesions from the MGH OCT registry who underwent planned follow-up OCT examination at 6 or 12 months after SES implantation from August 2010 to November 2013. From these patients, we excluded 6 cases with poor OCT image quality. Ultimately, a total of 140 patients comprising 181 stents were included in the study. Subjects were divided into 3 groups: current smokers (32 SESs in 28 subjects), former smokers who had quit at least 3 months before (50 SESs in 35 subjects), and those who had never smoked (99 SESs in 77 subjects). Drug-eluting stent (DES) implantation was performed using conventional techniques and most patients received dual (aspirin and clopidogrel) antiplatelet therapy for at least 12 months. The study protocol was approved by the institutional review board of each site, and written informed consent was obtained from all patients. The MGH OCT Registry is registered on ClinicalTrials.gov ( NCT01110538 ).
Coronary angiograms were analyzed using off-line software (CAAS 5.10.1; Pie Medical Imaging BV, Maastricht, The Netherlands). Diameter stenosis, reference diameter, and minimum lumen diameter were measured. Angiographic restenosis was defined as a diameter stenosis >50% at follow-up angiography.
The time-domain OCT system (M2/M3 Cardiology Imaging System; LightLab Imaging, Inc., Westford, Massachusetts) or the frequency-domain OCT system (C7-XR OCT Intravascular Imaging System; St. Jude Medical, St. Paul, Minnesota) were used in this study. The methods of intracoronary OCT imaging have been described previously. All OCT images were stored digitally, de-identified, and submitted to the MGH laboratory for off-line analysis. All cross-sectional images were initially screened for quality assessment and excluded from analysis if a side branch occupied >45° of the cross-section, if any portion of the stent was out of the screen, or if the image had poor quality caused by artifact, residual blood, or reverberation.
Cross-sectional OCT images were analyzed at 1-mm intervals. Stent and luminal cross-sectional areas (CSAs) were measured, and neointimal hyperplasia CSA was calculated as the stent CSA minus the luminal CSA. Mean values were reported in this study. The thickness of neointimal hyperplasia was measured as the distance between the endoluminal surface of the neointima and the strut. An uncovered strut was defined when no material covering a strut was identified ( Figure 1 ). The percentage of uncovered, protruded, or malapposed struts in each stented lesion were calculated as the (number of uncovered, protruded, or malapposed struts/total number of struts in all cross-sections of the lesion) × 100, respectively. A protruded strut was defined as any strut being in contact with the vessel wall but with <1/2 strut thickness impacted into the vessel wall ( Figure 1 ). A malapposed strut was defined as a strut that had detached from the vessel wall (≥160 μm; Figure 1 ).
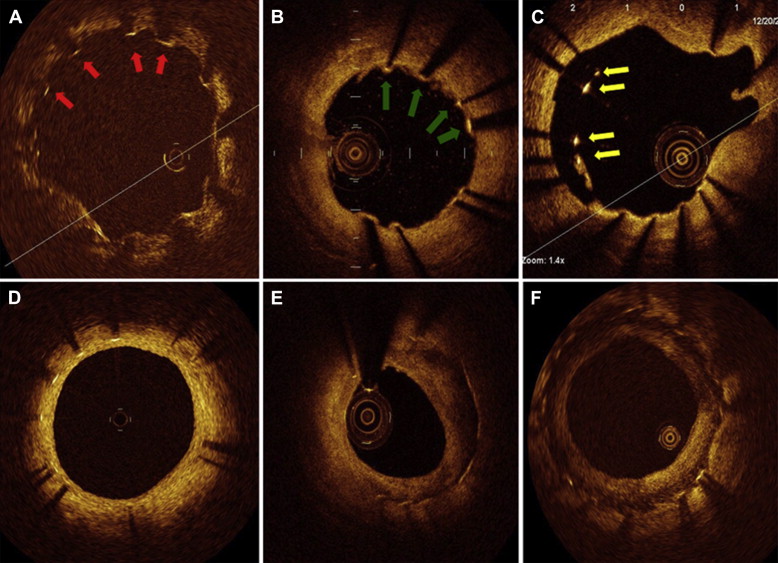
Neointimal tissue was classified as follows: (1) homogeneous neointima (a uniform signal-rich band without focal variation or attenuation), (2) heterogeneous neointima (focally changing optical properties and various backscattering patterns), and (3) layered neointima (tissue composed of concentric layers of a high-scattering endoluminal layer and a low-scattering abluminal layer delineated by a clear border) ( Figure 1 ). Thrombi were masses protruding into the vessel lumen with a dimension >250 μm.
OCT images were analyzed by 2 independent investigators blinded to patient information using OCT off-line software (LightLab Imaging). When there was discordance between the readers, a consensus reading was obtained from a third independent investigator.
For the description of subject demographic and clinical characteristics, categorical data were expressed as counts and percentages and compared using a chi-square test or Fisher’s exact test, depending on the distribution of the data. Continuous measurements were presented as mean ± SD and compared using analysis of variance followed by post hoc tests with Bonferroni correction for multiple comparisons. To determine the independent predictors of the presence of strut uncoverage and heterogeneous pattern neointima, multiple logistic regression analysis was performed for which the inference was made by generalized estimating equations approach with exchangeable correlation was used to account for the within-subject correlation arising from multiple stents within a single patient. Variables with p <0.1 from univariate analyses were entered into the final multiple logistic model. For the inference, interobserver and intraobserver reliabilities were estimated by means of the κ coefficient for binary outcomes and intraclass correlation coefficient for continuous measurements. All statistical analyses were performed with SPSS 17.0 (SPSS, Inc., Chicago, Illinois). For the multiple comparisons among the 3 groups, Bonferroni’s correction was applied; thus, a p <0.017 was considered statistically significant.
Results
Clinical characteristics of this study population are presented in Table 1 . Current smokers were significantly younger than nonsmokers or former smokers. Compared with nonsmokers, higher proportion of men and acute coronary syndrome were found in the current smokers. In the follow-up angiography, in-stent percent diameter stenosis, minimum lumen diameter, and in-stent restenosis were not different among the 3 groups ( Table 2 ).
| Characteristic | Cigarette Smoking | Pearson Chi-Square or ANOVA | p value CS vs. NS | p value CS vs. FS | p value FS vs. NS | ||
|---|---|---|---|---|---|---|---|
| Current (n=28) | Former (n=35) | Never (n=77) | |||||
| Age (years) | 51.1 ± 7.7 | 57.5 ± 11.6 | 59.6 ± 9.5 | <0.001 | <0.001 | 0.034 | 0.918 |
| Men | 26 (93%) | 30 (86%) | 44 (57%) | <0.001 | <0.001 | 0.448 | 0.003 |
| Hypertension | 13 (46%) | 23 (66%) | 48 (62%) | 0.247 | N/A | N/A | N/A |
| Hyperlipidemia | 21 (75%) | 29 (83%) | 66 (86%) | 0.436 | N/A | N/A | N/A |
| Diabetes mellitus | 8 (29%) | 14 (40%) | 31 (40%) | 0.526 | N/A | N/A | N/A |
| Creatinine (mg/dl) | 0.9 ± 0.1 | 1.0 ± 0.3 | 0.9 ± 0.2 | 0.614 | N/A | N/A | N/A |
| Total cholesterol (mg/dl) | 167.4 ± 45.1 | 153.3 ± 37.4 | 149.7 ± 41.4 | 0.376 | N/A | N/A | N/A |
| LDL-cholesterol (mg/dl) | 92.4 ± 30.4 | 90.3 ± 32.7 | 82.0 ± 31.2 | 0.815 | N/A | N/A | N/A |
| Triglycerides (mg/dl) | 124.0 ± 37.6 | 127.0 ± 32.6 | 99.0 ± 29.0 | 0.821 | N/A | N/A | N/A |
| Prior myocardial infarction | 6 (21%) | 14 (40%) | 24 (31%) | 0.287 | N/A | N/A | N/A |
| Prior coronary bypass | 1 (4%) | 0 | 0 | 0.133 | N/A | N/A | N/A |
| Left ventricular ejection fraction | 62.3 ± 7.7 | 61.9 ± 9.3 | 62.0 ± 7.0 | 1.000 | N/A | N/A | N/A |
| Clinical presentation at stenting | |||||||
| Acute coronary syndrome | 18 (64%) | 10 (29%) | 32 (42%) | 0.016 | 0.048 | 0.006 | 0.212 |
| Stable angina pectoris | 10 (36%) | 25 (71%) | 45 (58%) | 0.016 | 0.048 | 0.006 | 0.212 |
| Medications at follow-up | |||||||
| Aspirin | 27 (96%) | 33 (94%) | 72 (93%) | 0.850 | N/A | N/A | N/A |
| Clopidogrel | 26 (93%) | 25 (71%) | 57 (74%) | 0.082 | N/A | N/A | N/A |
| Statin | 27 (96%) | 31 (89%) | 71 (92%) | 0.515 | N/A | N/A | N/A |
| β-Blocker | 11 (39%) | 21 (60%) | 31 (40%) | 0.119 | N/A | N/A | N/A |
| ACE-I/ARB | 4 (14%) | 14 (40%) | 22 (29%) | 0.080 | N/A | N/A | N/A |
| Characteristic | Cigarette Smoking | p value CS vs. NS | p value CS vs. FS | p value FS vs. NS | ||
|---|---|---|---|---|---|---|
| Current (n=32) | Former (n=50) | Never (n=99) | ||||
| Coronary artery stented | ||||||
| Left anterior descending | 13 (41%) | 20 (40%) | 48 (48%) | 0.542 | 1.000 | 0.385 |
| Left circumflex | 9 (28%) | 13 (26%) | 20 (20%) | 0.340 | 1.000 | 0.531 |
| Right | 10 (31%) | 17 (34%) | 31 (31%) | 1.000 | 1.000 | 0.853 |
| Stent diameter (mm) | 2.9 ± 0.5 | 3.1 ± 0.5 | 2.9 ± 0.4 | 1.000 | 0.199 | 0.013 |
| Stent length (mm) | 24.1 ± 7.3 | 21.3 ± 7.2 | 23.4± 6.7 | 1.000 | 0.225 | 0.230 |
| QCA at Follow-up | ||||||
| Diameter stenosis | 12.2 ± 7.1 | 10.8 ± 6.5 | 12.4 ± 6.9 | 1.000 | 0.315 | 0.324 |
| Minimum lumen diameter (mm) | 2.4 ± 0.7 | 2.3 ± 0.7 | 2.3 ± 0.7 | 1.000 | 0.731 | 0.345 |
| Reference diameter (mm) | 3.2 ± 0.8 | 3.2 ± 0.6 | 3.1 ± 0.7 | 0.876 | 1.000 | 0.231 |
| In-stent restenosis | 3 (9%) | 4 (8%) | 8 (8%) | 0.729 | 1.000 | 1.000 |
The mean follow-up duration between stent implantation and OCT procedure was similar among the 3 groups (9.3 ± 2.7 months in current smokers, 9.7 ± 2.7 months in former smokers, and 9.0 ± 2.7 months in nonsmokers). All OCT images at follow-up were acquired successfully without any complications. Results of strut-level, cross-section–level, and stent-level OCT analyses stratified according to smoking status are presented in Table 3 . There were no significant differences in the mean neointima thickness among the 3 groups. The incidence of uncovered stent struts was significantly lower in current smokers compared with nonsmokers. No difference was observed in the incidence of malapposed struts or protruding struts among the 3 groups. Cross-section–level analysis showed that the cross-sections with uncovered struts and with an uncovered strut ratio >0.3 were significantly less frequent in current smokers compared with nonsmokers, although stent-level analysis showed no difference in the proportion of stents with ≥5% and ≥10% uncovered struts among the 3 groups ( Figure 2 ). The prevalence of heterogeneous neointima was higher in current smokers than in former smokers or nonsmokers. The frequency of homogeneous neointima was lower in current smokers than in nonsmokers ( Table 3 ).
| Variable | Cigarette Smoking | p value CS vs. NS | p value CS vs. FS | p value FS vs. NS | ||
|---|---|---|---|---|---|---|
| Current | Former | Never | ||||
| Strut-level analysis | ||||||
| Number of analyzed strut | 4,964 | 7,457 | 15,775 | |||
| NIT of covered struts (μm) | 120.0 ± 60.0 | 120.0 ± 60.0 | 130.0 ± 80.0 | 0.897 | 0.769 | 0.933 |
| Uncovered struts (%) | 6.7 ± 8.3 | 8.9 ± 10.2 | 13.3 ± 13.3 | 0.001 | 0.344 | 0.030 |
| Protruding struts (%) | 11.0 ± 13.8 | 11.4 ± 16.0 | 9.8 ± 12.9 | 0.693 | 0.927 | 0.619 |
| Malapposed struts (%) | 0.5 ± 2.2 | 0.8 ± 2.0 | 0.7 ± 2.2 | 0.176 | 0.582 | 0.785 |
| Cross-section level analysis | ||||||
| Number of analyzed frame | 653 | 867 | 1877 | |||
| Struts per cross section | 7.7 ± 1.4 | 8.5 ± 1.6 | 8.5 ± 1.7 | 0.010 | 0.016 | 0.894 |
| Mean luminal area (mm 2 ) | 6.5 ± 2.6 | 7.1 ± 2.3 | 6.1± 2.3 | 0.331 | 0.495 | 0.046 |
| Mean stent area (mm 2 ) | 7.7 ± 3.0 | 8.6 ± 2.0 | 7.2 ± 2.3 | 0.398 | 0.735 | 0.171 |
| Mean neointimal area (mm 2 ) | 1.3 ± 0.6 | 1.2 ± 0.8 | 1.2 ± 0.7 | 0.424 | 0.967 | 0.489 |
| Cross sections with uncovered struts (%) | 23.1 ± 27.1 | 32.8 ± 30.8 | 37.2 ± 29.1 | 0.010 | 0.141 | 0.373 |
| Cross sections with uncovered struts > 0.3 (%) | 8.5 ± 12.5 | 13.7 ± 22.1 | 18.4 ± 23.5 | 0.003 | 0.183 | 0.268 |
| Cross sections with malappositon (%) | 2.5 ± 9.7 | 3.7 ± 8.9 | 3.3 ± 10.0 | 0.142 | 0.362 | 0.647 |
| Stent-level analysis | ||||||
| Number of stent | 32 | 50 | 99 | |||
| Cross sections analyzed per stent | 20.4 ± 6.2 | 17.3 ± 7.3 | 19.0 ± 7.4 | 0.288 | 0.043 | 0.219 |
| Struts analyzed per stent | 155.1 ± 48.0 | 149.1 ± 72.5 | 159.3 ± 64.7 | 0.812 | 0.554 | 0.403 |
| Uncovered struts, stent with | ||||||
| At least 10% uncovered struts | 9(28%) | 18(36%) | 46(46%) | 0.053 | 0.468 | 0.191 |
| At least 5% uncovered struts | 14(44%) | 25(50%) | 68(69%) | 0.020 | 0.653 | 0.032 |
| Homogeneous signal neointima | 5(16%) | 13(26%) | 50(51%) | 0.002 | 0.264 | 0.014 |
| Heterogeneous signal neointima | 23(72%) | 18(36%) | 10(10%) | <0.001 | 0.004 | <0.001 |
| Layered signal neointima | 2(6%) | 1(2%) | 2(2%) | 0.257 | 0.353 | 0.998 |
| Thrombus | 3(9%) | 3(6%) | 3(3%) | 0.164 | 0.506 | 0.551 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


