Fig. 1.1
Number of transplants and size of active waiting list
This rise is attributable to the increasing number of waiting patients bridged with mechanical circulatory support devices . In the USA, approximately one-third of patients transplanted are actively supported by left ventricular assist devices. In some communities in the USA, 100 % of patients transplanted are supported by ventricular assist devices.
In an attempt to expand the donor pool, the mean age of donors has increased. In the USA, the mean age of donors has risen from 25 years in 1988 to 27 years in 2010. In Europe, the mean age of donors is now approximately 41 years of age. Concomitant with the attempt to expand the donor pool and increasingly supporting patients with ventricular assist devices, the survival of UNOS Status I candidates on the US Heart transplant waiting list has gradually improved. The survival at 12 months of UNOS Status I candidates from 1990 to 1994 was 16 %, from 1995 to 1999 survival has risen to 28.5 %, and during the early part of the current era from 2000 to 2005 it has risen to 40.2 % (Fig. 1.2). Of that surviving group, 23 % were on mechanical circulatory support systems. Of those transplanted, survival at 1 year was 84–87 %.
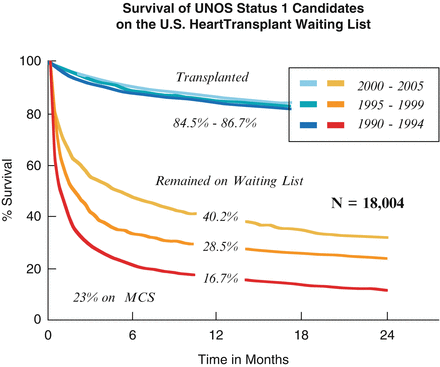

Fig. 1.2
Survival of UNOS Status I candidates on the US Heart transplant waiting list
The survival of UNOS Status II candidates has also improved (Fig. 1.3). From 1990 to 1994, the 12-month survival rate was 65 %, from 1995 to 1999 it was 72 %, and from 2000 to 2005 it was 80 %. Ninety percent to 93% of those patients transplanted survived at 1 year [5].
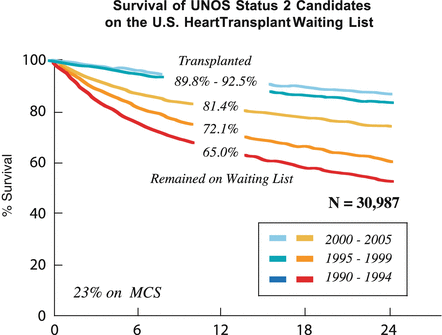

Fig. 1.3
Survival of UNOS Status II candidates on the US Heart transplant waiting list
The improvement in survival of medically treated Status II candidates now rivals the 1-year survival of the transplanted patients. Thus, the primary indication for transplantation of Status II patients is to improve their quality of life. The survival of heart transplant recipients has also slowly improved by era. The half-life in the decade from 1982 to 1992 was eight and one-half years, from 1993 to 2002 it was 10.9 years, and from 2000 to 2006 the survival was slightly improved; although half-life has not yet been reached, survival at seven years is approximately 64 %. The survival at 2 years has improved from approximately 70 to 81 % over the time period described (Fig. 1.4).
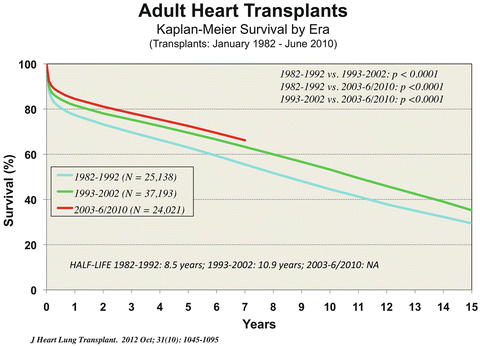

Fig. 1.4
Kaplan-Meier survival by era, adult heart transplants
The pivotal Rematch Study compared an optimally managed medical group before the era of resynchronization therapy to the survival of a group implanted with a pulsatile HeartMate I LVAD. The prospective control trial showed a dramatic improvement in survival for the LVAD arm, from 8 % to approximately 25 % at 2 years [6]. Subsequent trials comparing the HeartMate II continuous flow pump to the pulsatile HeartMate I predecessor demonstrated improved survival of 68 % at 2 years [7]. Individual centers now report 2-year survival of 80 % with ventricular assist device-supported patients [8]. This rivals transplantation at a 2-year time period.
No randomized trials currently exist comparing transplant patients to medical therapy or to long-term LVAD therapy, so all conclusions in this domain are based on comparative observations. Nonetheless, the 2-year efficacy for using LVADs to treat terminal heart failure has been proven. And now, initial explorations are being made trying to assess whether LVADs for destination therapy are on track to compete with heart transplantation. As recently reported by Kirklin [9], certain important subsets of patients, comprising 20 % of patients receiving continuous flow destination therapy, now enjoy a 2-year survival competitive with heart transplantation. The worldwide clinical experience of left ventricular assist device has increased dramatically and in 2013 over 14,000 patients have been implanted with a variety of devices. Currently, most patients have been implanted with the HeartMate II continuous flow pump. The increased global distribution of ventricular assist device implantations is tacit endorsement of the technology’s efficacy. In 2005, the USA, Canada, and five countries in Europe were implanting devices. The devices have now been implanted on every major continent, including South America, India, Eurasia, Asian Pacific, and Australia.
The estimated potential number of patients with ventricular assist device implants under the age of 70 in the USA is about 30 per 100,000. It has been further estimated that about 7 cases per 100,000 in the USA are added annually [10]. Comparing 2010–2011, in the 20 largest metro systems in the USA, the number of patients implanted is far below these projected numbers [11].
In summary, the heart failure landscape in 2013 shows a continued universal organ shortage and improved medical outcomes. There have been slight improvements in transplant outcomes and new rotary pumps provide improved 1- and 2-year survival. Their acceptance is increasing but has so far been less than projected.
1.2 Current Opportunities for Improvement
1.2.1 The Problem
There is a disparity between estimated need for LVADs and their actual application. The gap is likely due to the perceptions of quality of life, especially as influenced by real and perceived mortality , morbidity , and costs.
We analyzed the costs of transplantation in our own center and compared them to LVAD insertion. It was found that the cost of LVAD insertion declined by 40 % over a 5-year study period, while the cost of heart transplantation rose 12 %. Half of the LVAD implantation cost was the cost of the device. Interestingly, the causes of death for the LVAD population were largely a progression of other diseases. In contrast, the deaths in the transplant population were mostly attributable to immune-related maladies, often induced, such as neoplasm , infection , and graft rejection .
The importance of quality of life has been addressed by many and in the article by Stewart [12] about 60 % of patients with heart failure, quality of life was as important as survival. About 25 % felt that the quality of life was more important than survival and a few felt survival was more important than quality. In general, younger patients do prefer to live longer and older patients prefer to live better. Stewart determined what thresholds for physical activity and life expectancy currently prompt patients to consider an LVAD destination device. About 70 % of patients who felt that their life expectancy was about 8 months would consider themselves to be eligible for a left ventricular assist device. When asked “would you consider an assist device to be more active?,” 70 % of patients would consider an assist device if they could not dress themselves (Fig. 1.5).
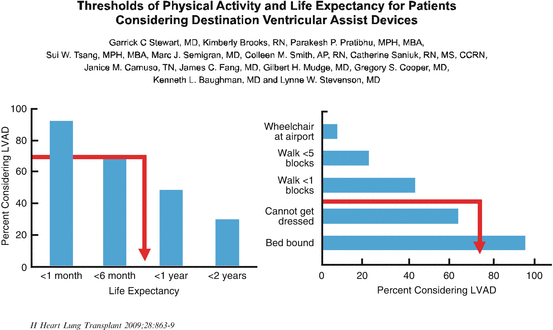

Fig. 1.5
Thresholds of physical activity and life expectancy for patients considering destination ventricular assist devices
Over the reported 2-year time course of LVAD implant studies, functional capacity and quality of life do improve. The six-minute walk distance, New York Heart Association Functional Classification , metabolic equivalent task score, Minnesota Living with Heart Failure Score, Kansas City Cardiomyopathy Score, and neurocognitive function all improve significantly following implantation of a HeartMate II and HeartWare assist devices. NYHA functional class was determined by an independent clinician at the time points shown [13]. Improvements were statistically significant in both trials (p < 0.001) [14–19]. Despite these proven advantages, real problems continue to influence the quality of life for recipients. If an implantable device had no associated mortality or morbidity , acceptance thresholds would certainly lower.
Hospital readmissions do have an adverse effect on quality of life. Using INTERMACS database , Kirklin assessed the readmission rate for over 2,000 LVAD recipients [20]. About half the patients had no readmissions, 22 % had one readmission and many patients had multiple admissions (Fig. 1.6).
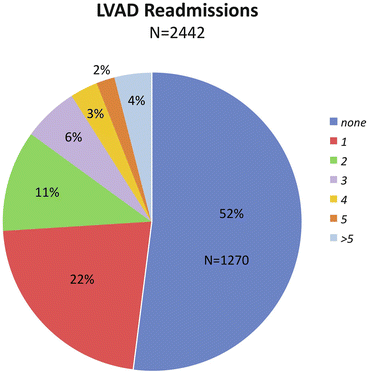

Fig. 1.6
LVAD readmissions
The most common cause was infection . The second most common cause was bleeding , followed by application of planned procedures such as pacemakers , ablations , and finally, a myriad of other problems including neurological, gastrointestinal, and pulmonary. Five percent of readmissions were due to right heart failure (Fig. 1.7).
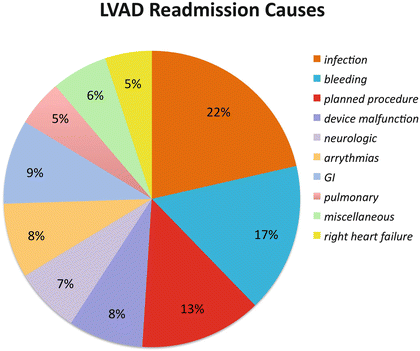

Fig. 1.7
LVAD readmission causes
The driveline infection curve shows a slope (Kirklin) that is continuous, reflecting a constant incidence of this problem (Fig. 1.8).
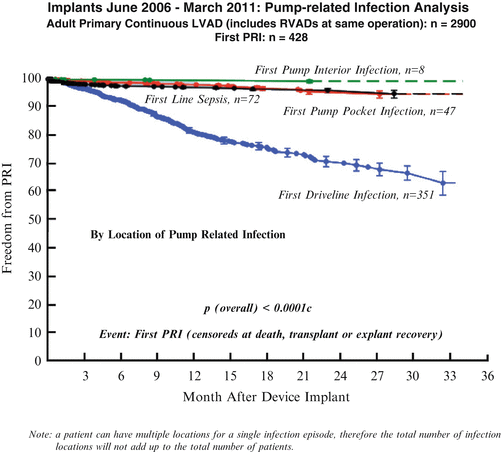

Fig. 1.8
Implants June 2006–March 2011: pump-related infection analysis
Overall, the occurrence of more serious internal pump infections , driveline sepsis , and pocket infections remain small. All are related to the liability of an implanted artificial surface.
1.3 Infection
1.3.1 The Solution
Standard definitions for infectious problems are being formulated in an attempt to ascertain the best way to prevent and treat them. Our own efforts have been published and have served as a reasonable guide for some [21]. The biomaterial interface is especially vulnerable as it passes through the skin where it is exposed to trauma and hostile bacteria. Future application of more bio-friendly surface technologies may help circumnavigate this problem. In the near future, fully implantable HeartMate II left ventricular assist device pumps will be available for use. A major advantage of fully implantable device systems is replacing the percutaneous drive line by using a transcutaneous energy transmission system . In order to achieve reasonable energy transmission efficiency these systems initially required close proximity of the inducting and receiving coils on and in the patient’s body. With the introduction of newer energy transmission systems, such as WiTricity , the devices are now able to be located more remotely in the body without severe loss of transmission efficiency. With the initial systems, superimposition of coils was important to achieve 80 % energy transmission efficiency. If the patients were to gain weight and increase the distance from the coils, the efficiency could be reduced to 50 %. Experience with these transcutaneous systems was reported during the early LionHeart implant fully implantable left ventricular assist device and the AbioCor fully implantable total artificial heart device . The current goal for a Thoratec is to have 3 h of untethered support time which will attenuate to 2 h after 2,000 cycles, representing approximately 2 years of recharges.
Using these systems will improve patient’s quality of life by allowing additional freedoms such as bathing and swimming. More importantly, in fully implantable systems, infection rates are expected to be reduced (Fig. 1.9).
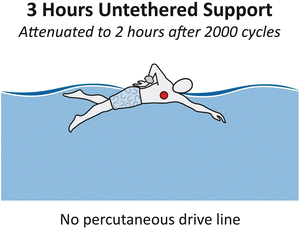

Fig. 1.9
Three hours untethered support—no percutaneous drive line
1.4 Thrombosis , Thromboembolism , Hemolysis , and Bleeding
Pump thrombosis, hemolysis, bleeding , and thromboembolism are all attributable to the inherent design characteristics of the continuous flow pumps . All are influenced by the interplay of recipient patient characteristics, implantation techniques, and management styles. There is no doubt that all of these hematologic liabilities are induced by the addition of an LVAD. Rotary continuous flow devices disturb the homeostatic state of the circulating blood by creating high shear stress levels and areas of non-laminar, even static flow conditions in the retained native heart.
Initial recommendations for anticoagulation and anti-aggregation strategies were made capriciously. Platelets were known to be activated by levels of shear stress exceeding 100 dynes. The high shear stress created by rotary pumps seemed to warrant the administration of antiplatelet drugs. Aspirin was recommended. Now, aspirin has been implicated in gastrointestinal bleeding in patients receiving coronary stents [22]. Furthermore, the well-documented loss of the high molecular weight von Willebrand multimer in rotary LVAD recipients creates platelet dysfunction. These facts bring into question the wisdom of using anti-aggregation medications for HMII.
Since stasis is known to occur with continuous flow conditions, anticoagulation with Coumadin seemed appropriate. This notion was reinforced by the high pump thrombosis rate of the early model of the MicroMed DeBakey-Noon pump [23].
Now, the routine use of Coumadin is being scrutinized. Certain subsets of patients seem to be more prone to bleeding than to thrombosis. In these patients, increased anticoagulation using Coumadin may be a liability. In our own experience of patients with bleeding abnormalities requiring cessation of anticoagulation, over 20 patients have fared well with no increased thrombotic complications for over 1 year.
The incidence of hemorrhagic and embolic strokes has been higher in females than in males. Younger patients seem to be more susceptible to thromboembolism. During the latter half of the continued access protocol for the HeartMate II, destination therapy trial experience anticoagulation levels were lowered. The incidence of hemorrhagic stroke was diminished, but the less morbid embolic stroke rate was not increased [24]. The origin of systemic embolization is often from the aortic outflow tract including the supra- and subvalvular regions and probably from embolic material generated within the pump.
The incidence of first stroke gradually increases with time. In the INTERMACS database at 36 months, 81 % of patients were free of stroke, and 92 % were free of pump thrombosis (Fig. 1.10).
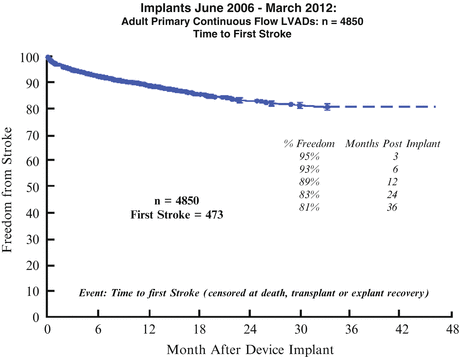

Fig. 1.10
Implants June 2006–March 2012: adult primary continuous flow LVADs
Pump thrombosis (Fig. 1.11) can be difficult to diagnose and the treatment and prevention has not been well defined. It currently felt that thrombosis can be the result of an ingested clot generated in the retained heart or by clot formed locally within the pump. Preventive strategies must address the coagulability of the individual host as well as the prothrombotic flow conditions that the pumps induce in themselves as well as in the host. The latter can be adversely influenced by odd apical cannula positions which restrict flow.
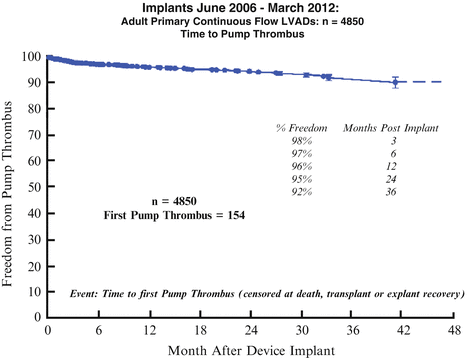

Fig. 1.11
Implants June 2006–March 2012: adult primary continuous flow LVADs; time to pump thrombus
Changes in current demands can be indicators of impending thrombosis if the thrombus creates drag on the rotor. Flow-restricting thrombus external to the rotor may not produce current changes. The use of flow sensors on rotary device conduits would be most helpful for diagnosing and predicting pump thrombosis. The additional information would hopefully serve as a guide for the more effective use of preemptive therapeutic lytic therapies.
Flow aberrations created by constricted areas of flow including clot formation in or around rotors can dramatically increase the rate of hemolysis in patients supported by continuous flow pumps . The hematologic cost of producing continuous flow is high. Flow architecture and recirculation, and high shear stress effects on blood elements, all function over time to create abnormalities. These abnormalities are tolerated to different degrees by different hosts. Since all left ventricular assist devices are recirculating devices, the flow history becomes more important over time [25]. The exposure to high shear stress history is dramatically increased in LVAD patients who have aortic insufficiency . This increases the instantaneous shear stress but also, by virtue of the central recirculation of blood, the flow history. Continuous flow pumps can be made to generate higher pressures, but they do so at the cost of increased shear stress. This may tax the hematologic shear stress tolerance of the individual host. This is dictated by the resilience of the formed elements (i.e., the presence of spherocytosis induced or acquired), hepatic and renal reserves coupled with the ability of the bone marrow to respond and compensate for reduced red blood cell survival times. When shear stress is over 2,000 dynes, red blood cells are destroyed. This hemolysis may be related to thrombosis since products of red cell lysis promote thrombin generation. Red cell survival studies in patients with bileaflet valves show that red cell survival is diminished from 120 to 100 days. In vivo red cell survival has not been measured to date with left ventricular assist devices but is surely low. Platelet activation occurs at greater than 50 dynes/cm2, with lysis over 100 dynes/cm2 and fragmentation over 250 dynes/cm2. Normal shear stress in biological systems ranges from 10 dynes/cm2 in the venous system to 70 dynes/cm2 in the systemic arterial circulation [26].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


