Operations on the Internal Carotid Artery
INDICATIONS FOR CAROTID SURGERY
The general adoption of the single metric of percent stenosis to select patients for intervention or operation is regrettable. It allows the treatment of some lesions that could be left alone and discards intervention in others that should be operated. Currently, it is generally accepted that in the absence of conditions that would increase the surgical risk or decrease life expectancy, endarterectomy should be considered in asymptomatic patients who have an ICA plaque causing a 70% to 99% stenosis. And having placed too much importance in this arbitrary boundary (70%) for intervention has probably resulted in inappropriate management of symptomatic patients with lesions of less than 70% diameter stenosis (with the usual pathologic features: hypoechoic plaque, ulceration, etc.) and unnecessary operations in asymptomatic, benign lesions causing a narrowing greater than 70% diameter.
And then, there are lesions that although clinically asymptomatic they present with ipsilateral “silent” infarcts seen in the CT (hence “signomatic”). The reason some infarcts are clinically silent is that the impaction of embolic material took place in an area that has no obvious clinical representation. Silent infarcts, however, result in the same long-term brain deterioration that is seen in infarcts that presented with TIA or stroke. It makes sense to apply to these signomatic lesions the same criteria for intervention that we use for symptomatic ones. There are two categories of patients with carotid lesions in whom the percent stenosis range limit should not apply: patients with “silent infarcts”1 because their plaques have already showed their embolic potential, and symptomatic patients (hemispheric/ocular TIA or stroke) who have a bifurcation plaque with the structural features (deep ulceration or surface thrombus) that cause embolization.
POSITIONING AND PROTECTIVE INTERVENTIONS
The patient is supine with a thin roll behind the shoulders and adequate support for the head so that at no time the latter is hyperextended or not fully supported. The head is turned away from the side of the operation. Before induction it is advisable to try the position of the neck in which the patient will be placed to detect the rare case where a patient cerebral perfusion depends on his VA supply and becomes symptomatic when the neck is turned and VA flow arrested.
The medication protocol for carotid endarterectomy includes the following: heparin 10,000 U IV, dextran 40, 100 mL push (plus 50 mL/h × 3), and dexametasone 10 mm IV.
As more patients are exposed to endovascular heparin from percutaneous diagnostics and interventions, the base dose of 10,000 U may not achieve the desired activated coagulation time of 300 seconds. If this is the case additional 5,000 U doses are given.
Dextran is a polysaccharide made of chains of glucose of different lengths and hence different molecular weights. Dextran 40, the one used in clinical medicine, is a low molecular weight dextran (40,000 Da) more easily excreted by the kidney than the heavier dextrans. Approximately 75% of dextran 40 is excreted within 24 hours of its administration. Dextran 40 increases the electronegativity of red blood cell and platelet membranes, decreasing their adhesiveness and platelet clump formation. The dose specified in this protocol has little risk of causing overload and cardiac failure. Anaphylaxis from dextran is rare and is even less common with low molecular weight dextrans.
In addition to using dextran IV at the time of heparinization, I use dextran topically to clear and wash the endarterectomy surfaces because once adsorbed onto the surface it will decrease platelet deposition on it when flow is resumed. Another advantage of using dextran to cleanse the endarterectomy surface is that it is a birefringent solution that, when the headlight is shown upon it, makes easy the identification of the small media debris that remains after the endarterectomy.
Dexamethasone given as a single 10 mg dose provides protection against an unknown allergy of the patient to X-ray dye or to dextran itself. But, more importantly, it decreases swelling secondary to brain ischemia. I am not aware of any downside of using a single 10 mg dose of dexamethasone during operations on the VA or CA.
In the 1970s much debate centered on whether or not a “shunt” should be used during carotid clamping, and whether there was a way of selecting those patients in whom the shunt would be necessary to avoid ischemia. There were futile attempts at trials and metaanalysis to sort out the significance of the use of a shunt in postoperative stroke amid the myriad variables involved in carotid operations.
I used a shunt routinely during the first 5 years of practice, then only in those patients with a contralateral occlusion and practically never in the last 15 years. During the time I used shunts I had the opportunity of observing some of the complications derived from the use of this presumably protective tool, such as intimal tears, short dissections, and atheromatous embolization from its proximal end “scooping” a grossly diseased CCA.
Those that advocate measuring the ICA “back-pressure” would insert a shunt when the back-pressure reading is <25 mmHg. With these criteria they end up inserting a shunt in 10% of patients. This is clearly an overuse since not using a shunt at all results in less than 1% of ischemic accidents in large personal series, including mine, and even in this fraction of 1% of patients, it is more likely that embolization from a technical flaw rather than low perfusion is the source of the infarction.
EEG monitoring complicates the operative setup and could only be of help if it could differentiate ischemia due to low perfusion rather than embolization. Actually, an embolus in the basal ganglia or occipital lobes will not be detected. A frontoparietal embolus, if detected, will surely stress the surgeon but its ischemic consequences will not reverse by inserting a shunt.
Doing the carotid endarterectomy under regional anesthesia and interrogating the patient periodically has also been used as the ultimate test for the need of a shunt. Again this results in an overestimation of the need for a shunt. In terms of cerebral blood flow one would expect a large decrease from 50 mL/100 g/min (normal) to 20 mL/100 g/min to cause unresponsiveness. But this level of relative ischemia, unless
prolonged, is not damaging to brain cells and function will return when perfusion returns to normal levels.i Furthermore, if a deficit arises the probability of it being secondary to microembolization is still high and no improvement can be expected in such a case from the insertion of a shunt. A shunt may be the source of potential problems in exchange for avoiding a rare mechanism of intraoperative stroke: critical hemispheric decrease in blood flow.
prolonged, is not damaging to brain cells and function will return when perfusion returns to normal levels.i Furthermore, if a deficit arises the probability of it being secondary to microembolization is still high and no improvement can be expected in such a case from the insertion of a shunt. A shunt may be the source of potential problems in exchange for avoiding a rare mechanism of intraoperative stroke: critical hemispheric decrease in blood flow.
HYPOTHERMIA IN “ARACHNIDS”ii
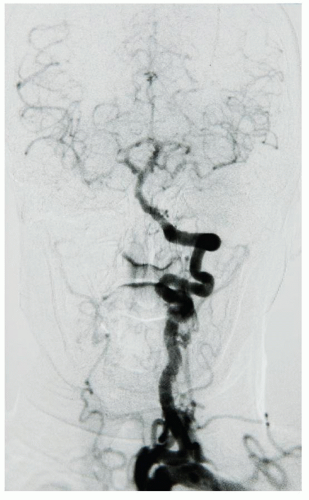
Most intraoperative brain infarctions are embolic, though some are the result of inadequate perfusion to a bed that has no adequate collateral supply. The longer the ischemic period, the more likely that neuronal death will occur. This situation may appear when operating on an isolated carotid that has no connection to the contralateral carotid (absent A1 and pCom) or, more commonly, when a patient who has a single remaining vessel directly supplying the brain, hence an “arachnid” (Fig. 5.1),
needs a reconstruction of this one vessel and there is no possibility of shunting. The rationale for using moderate hypothermia (33°C) when operating in these circumstances follows.
needs a reconstruction of this one vessel and there is no possibility of shunting. The rationale for using moderate hypothermia (33°C) when operating in these circumstances follows.
Brain damage caused by low flow or no flow is time dependent. A short arrest or severe decrease in flow, as we see in ventricular fibrillation or severe hypotension, does not result in neuronal death. Prolongation of the flow arrest impairs the blood-brain barrier, causes edema, suppresses cellular function (firing), and eventually damages cellular integrity. Damage will occur first in those tissues with the highest metabolic rate.
Hypothermia has a remarkable protective effect over the consequences of flow arrest. Different levels of hypothermia result in different protective effects. The time span between the ischemic insult and the state of hypothermia also determines the degree of protection achieved by the latter. Animal experiments have shown that protection is maximal if the ischemic insults occur when the brain has already been cooled. Conversely, the longer the delay between the ischemic insult and the cooling, the less protective effect the latter affords. If hypothermia is started beyond 2 hours after the ischemic insult, it has no protective effects.
With regard to the length of time that hypothermia needs to be maintained for neuroprotection the experimental evidence indicates that, when ischemia develops in an already cooled brain,iii the need for hypothermia may not be longer than 1 hour. This sequence of cooling prior to the start of ischemic injury fits well with the time sequence followed when using superficial hypothermia during operations on the ICA and VA: patients are cooled before clamping ischemia occurs.
There is solid experimental evidence that moderate hypothermia (33°C) results in a substantial decrease of cerebral metabolic rate (CMRO2) and a similar reduction in cerebral blood flow (CBF).2 It also reduces the inflammatory response of the brain tissue to infarction.3 It is estimated that for each 1°C decrease in core temperature there will be reduction of ±6% of the metabolic rate.
So, when the core temperature of a patient is lowered from 37°C to 33°C we should expect a 24% drop in metabolic needs. This will widen the time window under which the ischemic brain will be protected.
Mild and moderate hypothermia (35°C to 33°C) has been used in clinical studies to influence the outcome of an acute stroke. These studies used thermal blankets and jackets, bags of iced saline, and application of iced saline and alcohol to exposed skin. But these trials were marred by difficulties in dropping and maintaining the desired temperature, a problem compounded by heat generated by the shivering response in these awake patients.
In the surgical setting, the shivering response does not occur because cooling is not started until neuromuscular paralysis has been achieved during anesthesia. We use a cooling blanket and jacket as well as iced saline bags in the armpits and groins. Thin patients reach 33.5°C after approximately 30 minutes, but obese patients may require 1 hour or longer. When the temperature reaches 33.5°C cooling is stopped, the iced saline bags are removed, rewarming begins through the thermal blanket and jacket and the operating room ambient temperature is raised, regardless of the stage at which the surgeon is in the dissection. The core temperature (measured in
the bladder and in the larynx) will continue to drift down to 32.5°C to 33.0°C in the next 20 minutes. This is the time when the critical artery will be clamped and reconstructed. Rewarming will result in the temperature rising by about 2.0°C at the time of closure. Occasionally, after full reversal of heparin there may still be a slight increase in tissue oozing or suture hole bleeding because of remaining hypothermia. By the time the wound is closed, rewarming should continue and the patient is sent to recovery room on ventilator support not to be extubated until the core temperature reaches 36°C.
the bladder and in the larynx) will continue to drift down to 32.5°C to 33.0°C in the next 20 minutes. This is the time when the critical artery will be clamped and reconstructed. Rewarming will result in the temperature rising by about 2.0°C at the time of closure. Occasionally, after full reversal of heparin there may still be a slight increase in tissue oozing or suture hole bleeding because of remaining hypothermia. By the time the wound is closed, rewarming should continue and the patient is sent to recovery room on ventilator support not to be extubated until the core temperature reaches 36°C.
ACCESS TO THE CAROTID BIFURCATION
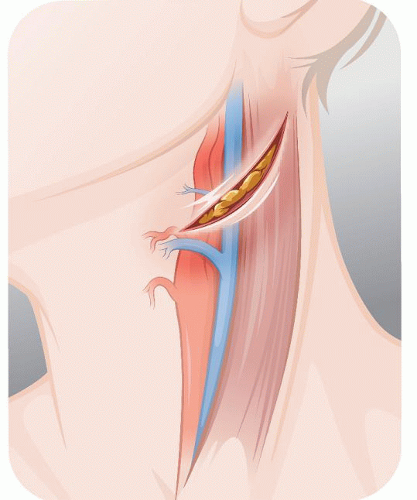
The incision, about 5 cm long, is oblique, almost transverse, curving around the angle of the jaw, 2 cm below it (Fig. 5.2). Once the platysma is divided and the external jugular vein (EJV) tied off, the dissection follows the anterior edge of the sternomastoid (SM) muscle. By lifting the proximal edges of the wound, the SM dissection is prolonged for another 4 to 5 cm below the edge of the wound so that later the SM can be retracted away from the jugular vein.
Rarely an unsuspected low bifurcation or a severely calcified CCA may require to expose the CCA lower for later clamping. To extend the incision inferiorly we follow the anterior line of the SM and divide the omohyoid muscle. The vagus tends to become anterior to the CCA as we dissect the latter towards the mediastinum. For this reason the dissection of the CCA begins in its anteromedial wall.
In the upper part of the incision we may encounter the auricular nerve. This nerve can be displaced upward by dividing the muscular roof of its tunnel (platysma) and mobilizing the nerve posteriorly.
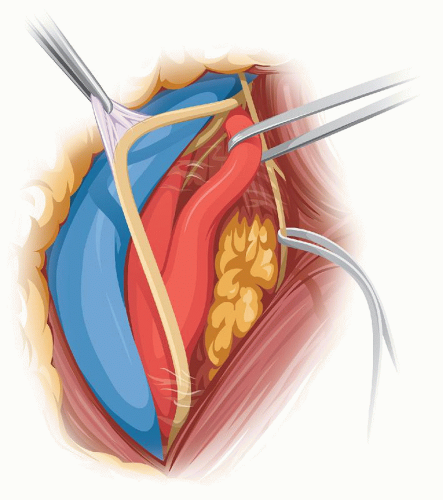
In individuals with a large parotid gland the lower tip of the superficial lobe is lifted from the SM and held inside the upper retractor. Distally, the tenddinous anterior edge of the SM is identified and the dissection follows on top of this tendon until the digastric muscle is met. The dissection of the internal jugular vein (IJV) starts at the bottom of the field and proceeds upward until the facial vein is identified and divided (Fig. 5.3). As the dissection continues on the anterior edge of the IJV, the next good-size vein that we encounter will be the one we call the “hypoglossal” vein because it usually covers the hypoglossal nerve (in a high bifurcation the facial vein may be the one covering the XII nerve). Before dividing this “hypoglossal” vein, we verify by that no underlying nerve is visible through the vein wall by tugging upward the right angle clamp and flattening the vein.
The CCA is isolated first bearing in mind the possibility that the vagus nerve may be anterior below the bifurcation. The vagus is dissected away from the CCA and this freeing of the nerve continues upward toward the top of the incision. Next the external carotid artery (ECA) and ICA are isolated. The superior thyroid artery is temporarily occluded with a hemoclip. During dissection of the superior thyroid artery or when placing the hemoclip, one should avoid damaging the flimsy external branch of the superior laryngeal nerve which frequently can be seen resting on the 1st cm of the superior thyroid artery (Fig. 5.4) (see Section “The Carotid Artery” in Chapter 1).
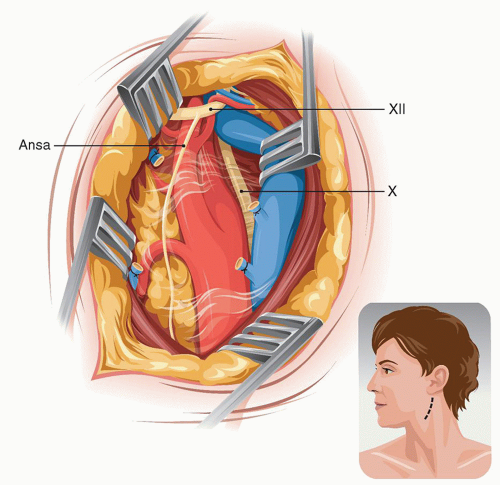 FIGURE 5.3. Posterior retraction of the IJV exposes the bifurcation. |
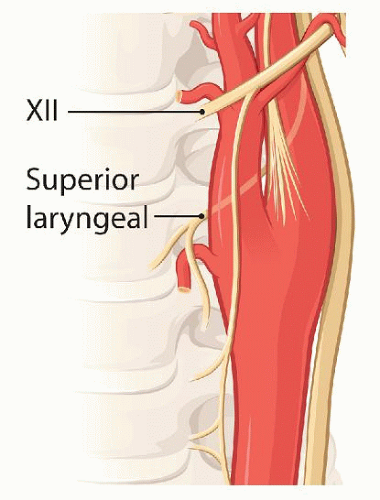 FIGURE 5.4. Relationship of the external branch of the superior laryngeal nerve to the superior thyroid artery. |
The ICA is dissected distally for as long as needed till a healthy gray-blue color in the entire perimeter of the artery indicates that there is no plaque at this level.
PROXIMAL EXTENSION
If the bifurcation is unexpectedly low or the quality of the distal CCA is not adequate for clamping (because of extensive calcification), the exposure needs to be prolonged proximally. If undermining the lower flap of the incision does not suffice, the skin incision is extended vertically.
DISTAL EXTENSION
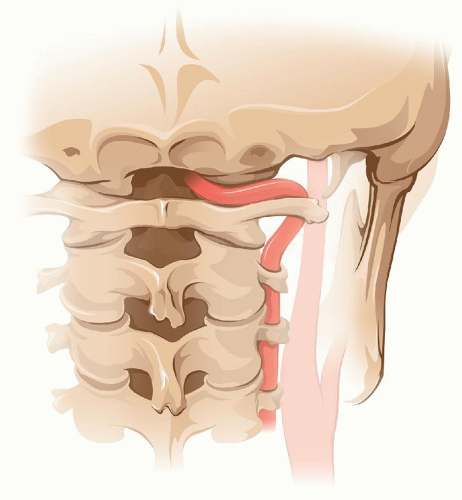
When the bifurcation is unusually high or when the distal end of the carotid plaque is above the level of the hypoglossal nerve, the exposure may need to be extended distally. The digastric muscle is first divided. Next the occipital artery (or its sternomastoid branch as may be the case) is divided as it loops over and tents down the hypoglossal nerve. Once the XII nerve is free it can be displaced anteriorly allowing more exposure of the ICA. If further length of distal ICA is required, the artery is approached above the XII. In this case it is of essence to avoid damaging the glossopharyngeal nerve as it crosses over the ICA. Once the hypoglossal nerve is mobilized and the ICA is dissected above it, an additional 2 cm of this artery can be freed.
If the plaque reaches the level of the hypoglossal nerve or higher, the best way to handle it is to transpose the ICA in front of the nerve (Fig. 5.5). For this, the ICA is divided at its origin, as if for a Type 1 eversion, and then pulled under and then over the hypoglossal nerve leaving the latter posterior to the artery. This allows straight traction of the ICA to free additional length distally. In this case, when reanastomosing the ICA, after the eversion endarterectomy, the artery is left anterior to the hypoglossal nerve.
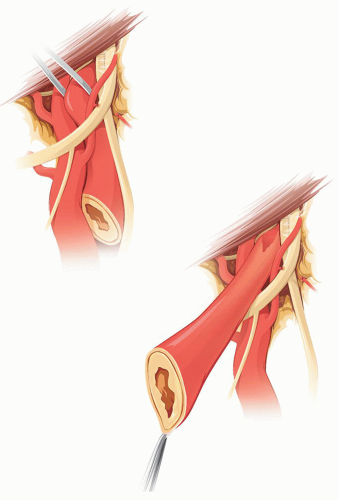 FIGURE 5.5. After severing its origin, transposition of the ICA in front of the XII nerve permits further distal dissection of the former. |
RETROJUGULAR ACCESS TO THE INTERNAL CAROTID ARTERY AT C2
The retrojugulariv approach permits exposing the ICA up to the C1-C2 level. If control of the ICA is needed above C1, then the anterior infratemporal approach (see below) should be chosen. The incision for the retrojugular approach is the same as that described for standard exposure of the carotid bifurcation (Fig. 5.6). The difference here is that the dissection starts behind the IJV between it and the SM (Fig. 5.5). The accessory spinal nerve is identified 2½ fingerbreadths below the mastoid tip. Once the nerve is isolated and looped to protect it, the ICA is exposed by retracting the IJV and the vagus nerve anteriorly (Fig. 5.7).
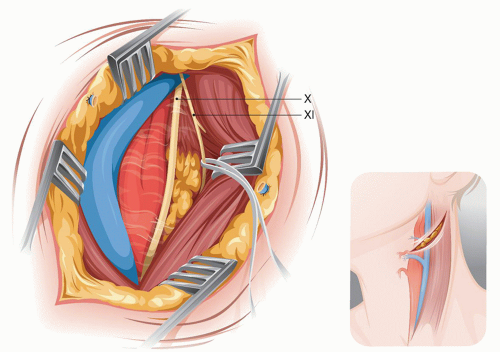 FIGURE 5.6. Retrojugular approach to the midcervical ICA. |
In this approach, the carotid bifurcation is exposed without the need to divide the facial vein. Above the bulb the ICA can be dissected without the encumbrance of the hypoglossal nerve that has been displaced anteromedially with the vagus and the IJV. The carotid can be dissected up to the takeoff (from the vagus) of the superior laryngeal nerve that crosses behind the ICA at the level of C2. Behind the exposed ICA, at the level of C2, lies the superior cervical ganglion.
DISTAL EXTENSION TO C1 (MANDIBULAR SUBLUXATION)
If the extent of the needed exposure had been anticipated to reach within 1½ cm below the temporal bone, we would have done a mandibular subluxation prior to the skin incision after the patient has been intubated naso-tracheally (Fig. 5.8). The subluxation is unilateral and should produce a 10 to 12 mm of underbite on the subluxated side. The mandible is maintained in place either with arch bars or with a wire loop through the anterior nasal spine and around the mandible.
The occipital artery and the digastric muscle are divided. The dissection proceeds above the XII nerve identifying the glossopharyngeal nerve that crosses over the ICA (Fig. 5.9). The IX nerve needs to be protected from traction or thermal injury that can result in disabling dysphagia. Just above the IX nerve the fingerpad of the surgeon rotated cranially palpates the sharp, bony styloid process. The latter is exposed with a key elevator and its distal two-thirds as well as the attached stylohyoid ligament are removed with a rongeur. The facial nerve is posterior to the styloid process and could suffer thermal injury if electrocautery is used at this level.
Distal control of the ICA is best achieved with a short balloon catheter (Fig. 5.10). The edges of the distal ICA are held with two sutures to facilitate reinserting a balloon in case of accidental pulling of the catheter or puncture of the balloon during the anastomosis.
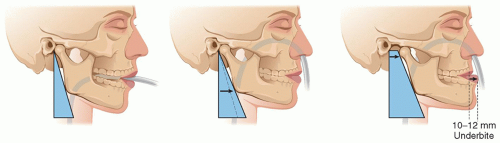 FIGURE 5.8. The relative contribution of endotracheal intubation (mid-frame) and subluxation (right frame) to the exposure of the distal ICA. |
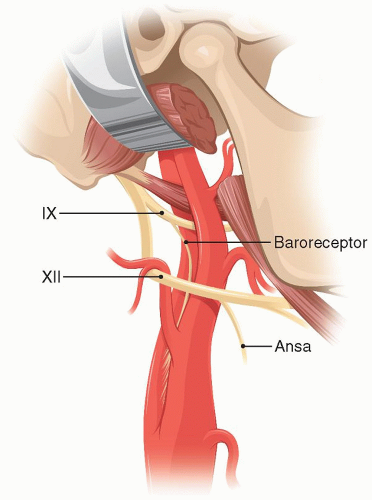 FIGURE 5.9. Schematic of the anatomy involved if the distal ICA is exposed. |
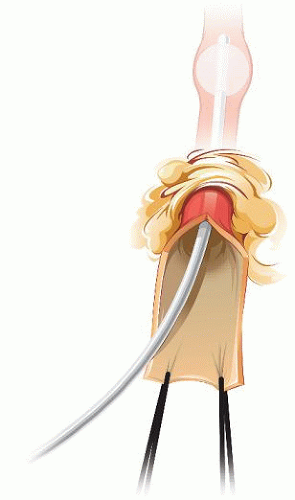 FIGURE 5.10. Distal control of the infratemporal ICA with a balloon catheter. |
POSTERIOR APPROACH TO THE INFRATEMPORAL INTERNAL CAROTID ARTERY
The infratemporal ICA can also be approached posteriorly. The upper cervical ICA, when approached from the posterior neck, lies in a trough whose bony sides are the cervical spine medially and the jaw laterally (Fig. 5.11). In this approach the mandible does not cover or obstruct access to the high cervical ICA. From the posterior
approach,v the infratemporal ICA is covered by the X, XI, and XII cranial nerves that need to be isolated and gently retracted away to mobilize the underlying carotid at this level (Fig. 5.12).
approach,v the infratemporal ICA is covered by the X, XI, and XII cranial nerves that need to be isolated and gently retracted away to mobilize the underlying carotid at this level (Fig. 5.12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree