Open Thoracoabdominal Aneurysm Repair
Ralph Bolman
Tsuyoshi Kaneko
Thoracoabdominal aortic aneurysm (TAAA) is pathologic dilatation of the thoracic aorta, traversing the diaphragm and extending into the abdominal aorta. The incidence is reported to be 10 per 100,000 person-years, and the two major causes of TAAA are atherosclerosis-induced degeneration and chronic aortic dissection. Hypertension, chronic obstructive pulmonary disease (COPD), smoking, obesity, hyperlipidemia, presence of aortitis, and connective tissue disorder (Marfan syndrome, Loeys–Dietz syndrome, etc.) are risk factors for the development of TAAA.
Surgery should be performed to avoid rupture or dissection. All symptomatic patients should undergo consideration of intervention, regardless of aneurysm size, as the presence of symptoms likely represents contained or impending rupture. The typical symptom for TAAA is back or abdominal pain. The severity of pain is often extreme, and in most patients is described as tearing or stabbing in nature.
Current American College of Cardiology and American Heart Association (ACC/AHA) guidelines for thoracic aortic disease gives Class I recommendation for asymptomatic TAAA repair for size over 6 cm, and less in patients with connective tissue disorder. The decision to intervene must involve risk-benefit analysis. Aortic diameter of 5.5 to 6.5 cm can be used as an indication for surgery, based on patient characteristics, institutional experience, and outcomes. The threshold for surgery should be lowered in patients with connective tissue disorder or family history of death from premature rupture of aneurysm. Other indications for surgery include growth of the aneurysm of 1 cm or more per year, compression of adjacent organs, acute dissection in an aneurysmal segment of aorta not amenable to endovascular repair, and impending or actual rupture.
Open TAAA repair is a highly invasive operation, and the patient must be able to tolerate this surgery. High-risk patients should be considered for medical management or hybrid or endovascular TAAA repair, therapies which are described elsewhere in this book. Although there are no absolute contraindications for surgery, patients with severe lung disease, severe left and/or right heart failure, chronic renal disease, cirrhosis, and age over 80 are considered relative contraindications. Presence of moderate or higher
aortic insufficiency will cause difficult-to-control left ventricular distention on extracorporeal circulatory support, and therefore also represents a relative contraindication.
aortic insufficiency will cause difficult-to-control left ventricular distention on extracorporeal circulatory support, and therefore also represents a relative contraindication.
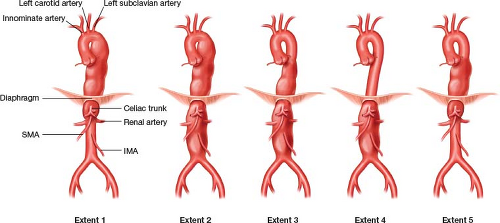
The Crawford classification of TAAA is the most commonly used classification, and aids the preoperative risk stratification and surgical planning (Fig. 8.1). Extent 1 originates from the left subclavian artery and ends above the renal arteries. Extent 2 is the most extensive form, extending from the left subclavian artery to the aortic bifurcation. This category entails the highest morbidity and mortality associated with surgical repair. Extent 3 extends from mid-thoracic aorta (T6) to the aortic bifurcation. Extent 4 starts below the diaphragm and extends to the aortic bifurcation. Safi’s group proposed an Extent 5, which is a subgroup of Extent 3. This category originates from the mid-thoracic aorta and extends to the level of the celiac and superior mesenteric artery (SMA), but ends above the renal arteries. Extent 5 is not recognized as a formal classification by the Society of Vascular Surgeons (SVS).
Computed tomographic angiography (CTA) with three-dimensional reconstruction is currently the gold standard for preoperative imaging. Magnetic resonance angiography (MRA) can be used as an alternative. Extent of the aneurysm, patency of the abdominal branches, presence of extra/accessory renal artery or large intercostal/lumbar arteries can be identified by thorough review of CTA.
Careful evaluation of pulmonary, cardiac, and renal function is important prior to embarking on open repair of TAAA. Pulmonary function tests and serum creatinine must be obtained. Patients with poor lung or renal function may best be considered for hybrid endovascular repair rather than open repair.
Transthoracic echocardiogram (TTE) should be obtained preoperatively to examine the left ventricular function and to assess the degree of aortic insufficiency. The incidence of coronary artery disease in TAAA is reported to be less than 30%, much lower than in abdominal aortic aneurysm, where the incidence is as high as 70%.
ACC/AHA guideline state that the data for preoperative coronary revascularization in descending thoracic aortic surgery is not well established. This derives from randomized control data showing no improvement in outcome with preoperative coronary revascularization. However, it is reasonable to obtain cardiac stress test preoperatively to look for reversible cardiac ischemia.
To avoid the main complications of open TAAA repair, such as renal failure, paraplegia, and mesenteric ischemia, perfusion strategy and adjunctive methods must be carefully planned and employed.
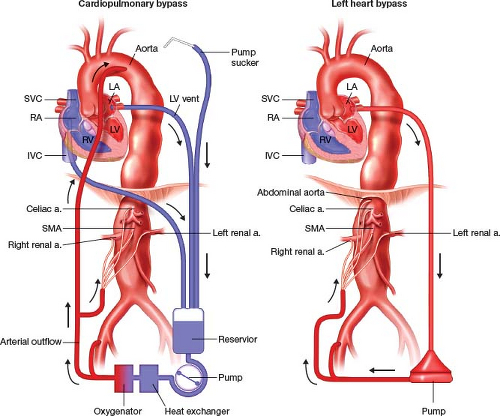
For perfusion strategy, either cardiopulmonary bypass (CPB) or left heart bypass (LHB) may be utilized (Fig. 8.2). These stratagems allow distal perfusion of the abdominal viscera, kidneys, lower extremities, and spinal cord while the cross-clamp is applied to the aorta in a stepwise fashion.
LHB is established by left atrial drainage through a left pulmonary vein or the LA appendage, and distal arterial perfusion into the abdominal aorta and to visceral branches. LHB requires a lower activating clotting time (ACT), unloads the left ventricle, and does not have an oxygenator in the circuit. LHB requires a double-lumen endotracheal tube, and mandates patient oxygenation by the dependent right lung throughout the procedure. A potential advantage of LHB is the avoidance of the membrane oxygenator, which can be the source of a systemic inflammatory response postoperatively.
Multiple papers from large institutions report safety of LHB for open repair of TAAA. These experiences notwithstanding, however, it is our preference to utilize CPB for the following reasons: 1. CPB allows more and more thorough cooling of the patient, especially when deep hypothermic circulatory arrest (DHCA) is necessary; 2. CPB also allows the use of cardiotomy suction, in order to minimize blood loss; 3. CPB allows reliable oxygenation of the patient throughout the procedure, without relying on the
dependent right lung. For all TAA repairs, we employ as a minimum moderate hypothermia for purposes of spinal protection. CPB is established with percutaneous venous drainage from the right atrium through a femoral vein, and arterial perfusion in both the distal arch and a femoral artery. The descending aorta may also serve as the site of arterial inflow. The arterial perfusion can be branched to allow perfusion of visceral branches. With cooling below 28 to 30°C, it is necessary to vent the left ventricle, in order to prevent LV distension with fibrillation. This is performed via a small incision in the pericardium, and direct venting of the LV apex. One exception to this is in patients with moderate–severe aortic insufficiency. These patients may either need to have the aortic valve replaced or undergo hybrid endovascular TAAA repair.
dependent right lung. For all TAA repairs, we employ as a minimum moderate hypothermia for purposes of spinal protection. CPB is established with percutaneous venous drainage from the right atrium through a femoral vein, and arterial perfusion in both the distal arch and a femoral artery. The descending aorta may also serve as the site of arterial inflow. The arterial perfusion can be branched to allow perfusion of visceral branches. With cooling below 28 to 30°C, it is necessary to vent the left ventricle, in order to prevent LV distension with fibrillation. This is performed via a small incision in the pericardium, and direct venting of the LV apex. One exception to this is in patients with moderate–severe aortic insufficiency. These patients may either need to have the aortic valve replaced or undergo hybrid endovascular TAAA repair.
Additional adjunctive methods include cerebrospinal fluid (CSF) drainage. CSF drainage is recommended for Extent 2 TAAA repair, given the high risk for paraplegia. ACC/AHA guidelines give a Class I recommendation for CSF drain, Class IIa recommendations for distal aortic perfusion, and for moderate hypothermia, and Class IIb recommendations for the use of glucocorticosteroids, mannitol, and motor/somatosensory-evoked potential monitoring.
Motor-evoked potential (MEP) monitoring is performed by excitation of motor neurons and measurement of peripheral muscle response. Changes in MEP suggest potential spinal ischemia. Somatosensory-evoked potential (SSEP) measures the sensory response in a similar fashion. Since the anterior horn of the spinal cord, which supplies the motor neurons, is more susceptible to ischemia than the posterior horn, which supplies the sensory neurons, changes in MEP are more sensitive for detection of ischemia compared to SSEP.
Intraoperative techniques such as sequential clamping of the aorta and reattachment of intercostal/lumbar arteries can be utilized to minimize the ischemic time to the visceral organs and the spinal cord.
Positioning
The CSF drain and electrodes for MEP and SSEP are placed prior to anesthetic induction. A double lumen endotracheal tube is inserted after induction. Antibiotics (cephalosporin and vancomycin) are given prior to incision. Transesophageal echocardiogram (TEE) is placed prior to repositioning.
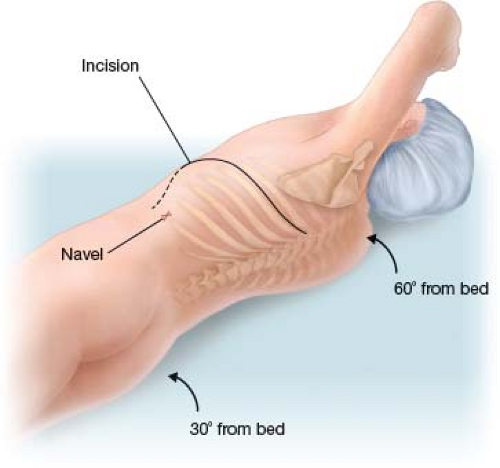
The patient is then placed in a right semilateral decubitus position (Fig. 8.3). The shoulders are turned 60 degrees, and the hip is turned 30 degrees so that the left groin is accessible.