Fig. 32.1
Open surgical repair of thrombosed superior mesenteric artery dissection (SMAD) with aneurysmal dilatation in a 78-year-old male who presented with abdominal pain. (a) SMAD on sagittal view of computed tomographic angiography; the aneurysm contains significant amount of mural thrombus proximally and completely thrombosed distally (arrow). (b) Intimal flap of SMAD (arrow) on 3-D reconstruction and moderate amount of peripheral calcification of SMA. (c) Intraoperative photograph of the SMAD with the artery and side branches on vessel loops. (d) Excision of SMAD, proximal and distal SMA interposition graft using 8 mm Hemashield Dacron graft, and reimplantation of four large jejunal branches
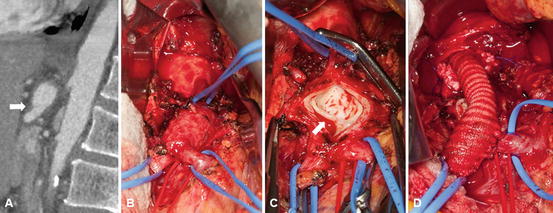
Fig. 32.2
Open surgical repair of celiac artery dissection (CAD) with aneurysmal dilatation in a 61-year-old male with progressive epigastric pain. (a) CAD on sagittal view of computed tomographic angiography; thrombosed proximal celiac artery with distal aneurysm with dissection (arrow). (b) Intraoperative photograph of the CAD with branches on vessel loops. (c) The CAD was opened. Note dissection flap in the artery (arrow). (d) Repair with aorto-distal celiac artery 8 mm Hemashield Dacron interposition graft with extension of the distal anastomosis onto the common hepatic artery
Many of the revascularization procedures are similar to those used for acute or chronic mesenteric ischemia or for atherosclerotic or true aneurysms of the visceral vessels. For detailed techniques of these procedures, the readers should consult Chaps. 11, 16, and 17, regarding open mesenteric revascularization.
Superior Mesenteric Artery Dissection (SMAD)
Surgical revascularization procedures have been performed using antegrade or retrograde aorto-SMA or a retrograde iliac artery-SMA bypass or an SMA-SMA interposition graft; bypass between the SMA and right gastroepiploic artery or transposition of SMA to the more distal aorta was also reported [12, 38, 46–49].
Dong et al. reported the technique of surgical fenestration of the SMA. The target segment was transected, the intimal flap of the proximal stump was excised, and the flap in the distal artery was reattached to the wall with sutures. The two stumps of the artery were then reanastomosed in an end-to-end fashion [38].
Celiac Artery Dissection (CAD) and Hepatic Artery Dissection (HAD)
The surgical procedure can be performed via an upper midline or chevron incision. The aneurysm is approached through the gastrohepatic ligament, the lesser sac is entered and the crus of the diaphragm divided if the aorta needs to be dissected, and a bypass is performed between the supraceliac aorta and the celiac trunk using Dacron or, in a patient with an infected field, with a vein graft [50, 51].
Renal Artery Dissection (RAD)
Open repair of RAD is a technically demanding procedure. Standard aortorenal bypass with vein or Dacron or autologous internal iliac artery is indicated in patients when the dissection only involves the main renal artery or a short segment of its primary branches [40, 53].
Extra-anatomic reconstruction (splenorenal or hepatorenal bypass) can also be performed. Ex vivo reconstruction in these patients is frequently required to salvage the ischemic kidney, treat hypertension, or prevent rupture of the artery. This technique is used in patients when vascular lesions are located more distally and extend into the secondary or tertiary branches of the renal artery [6, 40, 53]. Once exteriorized for the repair, the kidney is perfused with chilled Collins’ solution and packed with ice [6, 40]. In some patients, extracorporeal repair and autotransplantation of the kidney into the pelvis were reported [40, 53, 54].
Endovascular Revascularization
Nowadays, with improved interventional technologies and rapid development of endovascular materials, catheters, and stents, minimal invasive percutaneous endovascular treatment has become an attractive alternative to surgical revascularization for IVAD. Endovascular therapies include catheter-directed thrombolytic therapy, coil embolization, and placement of bare metal or covered stents. The specific application of endovascular treatment should be tailored to the needs of the patient, according to clinical presentation and the location and extent of dissection [14, 16, 27, 33, 59, 60].
Catheter-Directed Thrombolytic Therapy
Catheter-directed thrombolytic therapy of acutely thrombosed and dissected visceral arteries has been reported, with mixed results. Secondary procedures, including stenting [61] and laparotomy [2, 62], are frequently needed. Most success with catheter-directed thrombolysis (CDT) was achieved in patients with renal artery thrombosis due to dissection [42–44, 56]. In cases of RAD with acute renal artery thrombosis, CDT using urokinase [42, 43] and tissue plasminogen activator (rt-PA) [44], or combined with aspiration thrombectomy under antithrombotic protection with glycoprotein IIb/IIIa inhibitor [56], has been used successfully before stent placement in hemodynamically stable patients and achieved good early results.
Embolization
Sufficient collateral flow is essential to avoid organ infarction if we use embolization for IVAD to occlude visceral arteries. Successful embolizations of renal and celiac artery dissections have been reported [63]. Takeda et al. [60] reported on treatment of CAD with coils. Platinum-fiber coils (Cook Medical) and detachable Amplatzer Vascular Plugs (AVPs) I, II, and/or IV (AGA Medical, Golden Valley, MN, USA) were embolization materials used. Perini et al. recommend that the diameter of the AVPs is approximately 30–50 % larger than that of the treated artery [45].
Li et al. suggested coil embolization to occlude the false lumen and stent placement to improve flow through the true lumen in patients with type V SMAD [25]. However, the need for coil embolization in such patients has been questioned since placement of self-expandable stent will reduce the aneurysm size with gradual resolution of the false lumen and the increase in diameter of the true lumen [34, 38, 64, 65].
Stenting Visceral Arteries
At present, there are no evidence-based data that support the choice of the best type of stent for each specific IVAD. For principles of visceral artery stenting and the use of balloon or self-expandable stents, or covered or bare metal or nitinol stents, the readers should consult Chaps. 13, 14, and 18. Precise positioning and good flexibility are requirements for the stent used in the treatment of IVAD. Careful planning includes selection of the length of the stent that can be deployed at the location, the size of entry and reentry sites, measurement of the length of the broad-based pseudoaneurysm, and the severity of arterial stenosis [16] (Fig. 32.3).
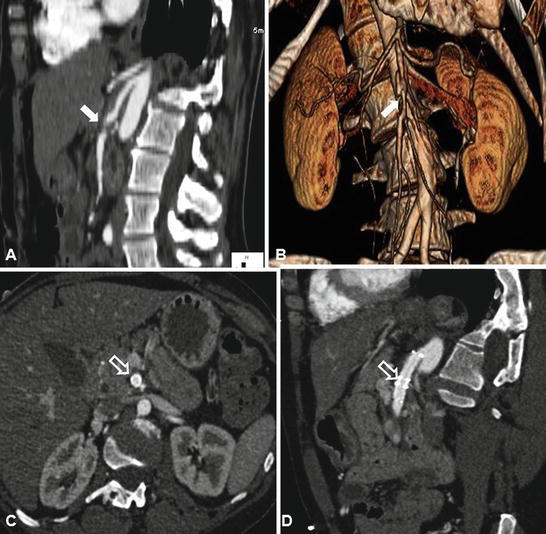

Fig. 32.3
Endovascular repair of partially thrombosed superior mesenteric artery dissection (SMAD) with self-expandable stents. (a) SMAD on sagittal view of computed tomographic angiography; the aneurysm contains significant amount of mural thrombus in mid SMA (arrow) with patent artery distally. (b) Intimal flap of SMAD (arrow) on 3-D reconstruction. (c) Axial view of computed tomographic angiography after stent placement in the SMA showed well-stented SMA (hollow arrow), and there was no evidence of intramural thrombosis. (d) On sagittal view of computed tomographic angiography, the involved SMA segment was fully covered by two stents (hollow arrow), and there was no evidence of intramural thrombosis, SMA stenosis, or compression (Courtesy of Timur P. Sarac, MD, Cleveland Clinic, Cleveland, OH)
Because of the radial strength, conformability, and sufficient length, self-expandable stents are recommended in the management of IVAD. Numerous stents such as WALLSTENT (Boston Scientific, Natick, MA), Zilver or Zilver 635® stent (Cook Medical, Bloomington, IN), SMART or PRECISE stent (Cordis, Miami Lakes, FL), Palmaz Blue stent (Cordis, Bridgewater, NJ), Xpert (Abbott Vascular, Abbott Park, IL), Astron Pulsar (Biotronik AG, Switzerland) have been used with diameters of up to 8 mm and overall lengths of up to 100 mm. Stents with a diameter 10 % greater than the diameter of the proximal artery are usually chosen [5, 16, 25, 31, 35, 58, 60, 66–68]. Sinus-SuperFlex nitinol stent (OptiMed, Ettlingen, Germany) was used with success by one group for RAD [56]. For short dissection, a balloon-expandable stent can be considered [33, 60]. However, the balloon-expandable stent is stiffer and the resulting axial force is greater so it should be avoided in the curved, second segment of SMA. Although covered stent was reported to treat a ruptured SMAD [26], a potential disadvantage is that it obliterates side branches of the treated artery. Covered stent, however, was also used with success to treat SMAD [69, 70].
Occasionally, laparotomy can be combined with transmesenteric endovascular approach and retrograde mesenteric stenting (hybrid treatment) [15, 49]. Intravascular ultrasound (IVUS) may be helpful to identify the true and false lumen, image the flap, and even establish the site of the tear in the artery.
The double-coaxial technique (mother-and-child technique) might be helpful to facilitate the delivery of stent to a complex lesion [71]. In patient with large discrepancy in diameters between proximal and distal arteries, two stents of different calibers with an overlap of 1.5 cm were suggested [14]. Chu et al. pointed out that in long dissection, coverage of a short segment at the entry tear by the stent graft or even bare stent can yield good result with gradual resolution of the mural hematoma on follow-up CTA images [16].
Currently, endovascular treatment with stenting is increasingly used in patients with RAD [43, 44, 56]. The risk of acute occlusion after stent placement, however, must be weighed in each case. Considering the lesion needs to be completely covered, long stents are frequently needed; however, long stents are more thrombogenic than short stents. Thus, stent thrombosis is also a concern. When the dissection extends into the renal hilum, the risk of renal infarct and further extension of the dissection with placement of a stent is high [56].
Conclusions
Treatment options for IVAD are largely dependent on the clinical presentation, acute or chronic symptoms, the location and extent of the dissection, and the size of the aneurysm. For asymptomatic patients and some mildly symptomatic patients with no or small aneurysm, conservative treatment with or without anticoagulation or antiplatelet medication is recommended; however, close follow-up with imaging studies using duplex ultrasound scanning or CTA is required to detect any progression of the dissection. Open or endovascular revascularization is indicated in patients who fail to conservative therapy or in those who present acutely with organ ischemia or infarcts, arterial occlusion without collateral circulation, or rupture. Because of the less invasive nature, endovascular procedure is considered first in all patients, although open surgery is still frequently needed to attempt to save the organ or the life of the patient if catastrophic complications occur.
References
1.
2.
3.
4.
5.
6.
Muller BT, Reiher L, Pfeiffer T, Muller W, Hort W, Voiculescu A, et al. Surgical treatment of renal artery dissection in 25 patients: indications and results. J Vasc Surg. 2003;37(4):761–8.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


