References
Dates
Device
#
Indication
Criteria
Data type
Statistics
El-Banayosy et al. [2]
1992–2000
Thoratec
104
BTT
>60
Bad Oeynhausen
OR 3.87
Dang (2001)
1993–1999
Novacor
464
BTT
>65
Registry
OR 3.01
Granfeldt et al. [4]
1993–2003
Several
59
BTT
Sweden
NS
Rao et al. [11]
1996–2001
HMI
130
BTT
49 ± 14
Columbia
NS
Dang et al. [5]
1996–2004
HMI
201
BTT
52 ± 12
Columbia
1.89/10 years
Topkara et al. [16]
1996–2004
HMI
201
>60
Columbia
No change with age
Huang et al. [7]
1996–2003
Novacor
222
>60
Registry
OR 1.97
Schenk et al. [14]
1991–2002
Several
207
Older age
Cleveland
P < 04
Holman et al. [6]
2006–2007
Several
420
50 vs. 60
INTERMACS
1.41
Sagrid (2009)
1998–2007
HMII
86
BTT
60
Vienna
Hazard 1.4
Schaffer et al. [13]
2000–2009
HMII
86
BTT
49 ± 13
Johns Hopkins
1.07
Zahr (2009)
1991–2005
BiVAD
44
BTT
Klotz et al. [9]
2003–2009
Several
241
BTT
>50
Muenster
OR 1.89
Leitz (2010)
1998–2005
HMI and II
377
DT
Registry
Center experience
Stepanenko et al. [15]
2006–2009
Several
28
DT
>65
Berlin
Significant
Kirklin et al. [8]
2006–2009
Several
1,092
Mixed
60–70
Registry
HR 2.42
A new era of MCS was ushered in by the development and subsequent FDA approval of the HeartMate II (HMII) continuous-flow LVAD [18–21]. With excellent durability, improved patient survival, decreased incidence of adverse events , and better patient satisfaction and quality of life, the HeartMate II was shown to be a superior option to the HeartMate I for both bridge to transplant (BTT) [18, 19] and destination therapy (DT) populations [20]. With an increasing population of elderly patients with advanced heart failure who have limited treatment options, there are unanswered questions pertaining to whether older patients can benefit from and are appropriate for this technology.
Congestive heart failure (CHF) is a common condition that increases with age. It is estimated that as many as 10 % of people over the age of 70 may be afflicted and as many as 150,000 experience class IV symptoms [22]. Medical management of this population is expensive and offers limited survival and potential for functional recovery . Cardiac transplantation has traditionally been the gold standard for comparing end-stage heart failure management but with a small donor pool (approximately 2,000 per year in the USA), and with the pragmatic restriction to patients under the age of 70 years, it appears that MCS will become the standard of care for older, refractory heart failure patients.
Patients with conditions such as advanced age , remote history of cancer, active infections , renal insufficiency , pulmonary artery hypertension, sensitization, and large body size especially with a common blood type could potentially be transplanted, but their waiting times are typically prolonged. Older patients (≥70 years) are the largest potential group that could benefit from LVAD support, yet advanced age has consistently been identified as a risk factor for poor outcome. As noted above, these studies have several limitations: (1) use of proven inferior technology (pulsatile devices), (2) registry data of LVADs in patients with diverse indications (i.e., failure to wean from CBP, deterioration while awaiting transplantation , and ongoing cardiogenic shock ), and (3) data from a variety of mixed low- and high-volume centers. Therefore, extrapolation from these earlier results may not accurately reflect the expected outcome with the newer continuous-flow HMII device. Hence, the main objective of our study [23] was to evaluate the outcomes of LVAD patients older than 70 years of age from a community hospital with an experienced VAD team.
3.2 Methods of Our Initial Evaluation of Elderly Compared to Younger Patients
3.2.1 Patient Inclusion Criteria
All patients studied met the clinical trial enrollment criteria and the general criteria for BTT/DT LVAD implantation as published by the Centers for Medicare and Medicaid Services (CMS) [23], including chronic end-stage heart failure (New York Heart Association [NYHA] class IV symptoms failing to respond to optimal medical management, end-stage left ventricular failure for at least 90 days, and a life expectancy of less than 2 years); left ventricular ejection fraction (LVEF) <25 %; demonstrated functional limitation with peak VO2 < 12 mL/kg/min; or continued need for intravenous (I.V.) inotropic therapy ; and an appropriate body size to support LVAD implantation [23].
3.2.2 Facility Criteria
Through a National Coverage Determination issued on October 2003, Medicare began coverage of the DT indication, and effective March 27, 2007, new facility criteria were established which require hospitals to receive certification from the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) [23]. Facilities gaining JCAHO certification are placed on a list on the CMS website, which is continuously updated [23, 24]. During the current study, our facility achieved and maintained JCAHO certification as a Medicare-approved VAD facility.
3.2.3 Preoperative Assessment and Clinical Optimization
The criteria adopted for selecting LVAD candidates are summarized in Table 3.2. All patients underwent comprehensive evaluation and treatment prior to LVAD placement, which included clinical assessment of the severity of heart failure , hemodynamic state , cardiac anatomy, and operative risk. Inotropic support, diuresis or ultrafiltration, infection surveillance/treatment, and nutritional assistance were provided when needed. Based on the response to treatment, it was determined if the patient should continue on medical treatment or hospice and offered high-risk surgical repair with LVAD standby or become a candidate for destination LVAD therapy or transplantation . For nonresponders the decision was whether they were appropriate LVAD candidates or too sick for support. Noncardiac considerations such as history of chronic or life-limiting illnesses, mental status, nutritional status, expectations (quality of life vs. duration of life), and psychosocial and age-related considerations were assessed.
Table 3.2
Criteria for selecting patients as LVAD candidates
Characteristics of patients selected for LVAD therapy | High-risk LVAD patients but not absolute contraindicators | Contraindications to LVAD support |
|---|---|---|
Intolerable congestive heart failure symptoms and/or lifestyle limitations despite maximal medical/surgical therapies | Severe chronic obstructive pulmonary disease (COPD) | Patient refusal |
Meets national standardized inclusion criteria | History of stroke with worsened neurocognitive defect secondary to congestive heart failure | Insufficient significant other support, home environment, or financial resources |
Adequate mental, psychological, social, and financial support to comply with the complex LVAD management protocols | Primary right heart failure (i.e., hypertrophic myopathy, arrhythmogenic right ventricular dysplasia, or posttransplant constrictive/restrictive disease) | Irreversible neurocognitive defects that preclude routine LVAD care |
A strong desire to have an LVAD implanted held by both the patient and his significant other caregivers | Fixed pulmonary artery hypertension | Ongoing cardiogenic shock refractory to all resuscitation measures including peripheral cardiopulmonary bypass (CPB) resuscitation |
Active infections | End-stage pulmonary disease out of proportion to congestive heart failure | |
Hepatic fibrosis or cirrhosis | Fungemia | |
Blood dyscrasias (heparin-induced thrombocytopenia, hypercoagulable states, current 2B3A drug use) | Ongoing drug or alcohol addiction or recent history of noncompliance to medical therapy | |
Renal failure including chronic dialysis dependence | ||
Patient or significant other ambivalence regarding the LVAD implant |
Neurological Assessment
A neurological evaluation was ordered if necessary to rule out degenerative central nervous system (CNS) diseases and dementia. Since it is imperative that candidates for LVAD therapy demonstrate the ability to operate the device safely, we assessed for stroke, psychiatric disorders, mental retardation, substance abuse, and other factors which could potentially impact compliance with medical regimen and clinic visit follow-ups [25].
Renal Assessment
Patient’s baseline renal function was reviewed since poor baseline renal function is associated with worse outcomes after LVAD implantation [26–28]. This included assessment of renal parameters as well as preoperative diuretics and aggressive treatment of heart failure (inotropic support, ultrafiltration, or dialysis).
Nutritional Assessment
For each patient, a comprehensive assessment was made to define the degree of malnutrition and estimate the severity of illness (SI). The Subjective Global Assessment (SGA) and SI scales were typically used to determine the level of malnutrition and to determine when to start nutritional support [29].
Psychosocial Assessment
When evaluating the patient’s social support network, the following were reviewed: the presence and age of the caregiver, such as a spouse or family member, who could be available in case of device malfunction; a plan of care for discharge which would ensure that the home environment was safe and allow the patient to receive adequate postoperative care; and the patient’s ability to care for himself, including eyesight, hearing, dexterity, and evidence of poor dentition, as well as any history of noncompliance with medical regimens. Social workers reviewed the availability of patient-related community resources. If significant questions were unanswered, then a trained home health nurse visited the patient’s home to complete the assessment.
Informed Consent
All patients received the information necessary to assist them in giving appropriate informed consent for the procedure. The patient and significant others signed a health-care contract delineating the expectations for aftercare.
Post–Op Management
Prevention of right heart failure was implemented in all patients, which included the routine use of inhaled nitric oxide, bi-ventricular pacing when necessary, and the use of inotropic drugs , such as isoproterenol, dopamine, and milrinone in the immediate postoperative period. Patients with impaired right ventricular function were given sildenafil routinely and the inhaled nitric oxide was weaned within 24–48 h in most cases. Nursing care in the cardiac step-down unit focused on strengthening, self-care, and education about LVAD management.
3.2.4 Patient Population
In our initial study [30], 55 consecutive patients receiving HMII LVADs included in the BTT or DT clinical trials from October 2005 through January 2010 at a small community hospital with extensive MCS experience were evaluated. Patients were divided into two groups based on age at the time of implant: Group 1, patients under the age of 70 (n = 25) and Group 2, patients 70 years or older (n = 30). During the study period, 329 patients were referred for consideration of LVAD or transplantation of which 15 % (49/280) were 70 years or older. The majority of patients, 61 % (30/49), 70 years of age or older, accepted and underwent HMII implant. Four patients (8 %) were considered to be good candidates but refused our recommendation; 16 % (8/49) were too well while only 8 % (4/49) were too ill. In the younger than 70 population 46 % (103/280) either received a HMII or were transplanted, 14 % (40/280) were too well, 5 % (13/280) underwent a traditional cardiac operation, 4 % (10/280) were too ill, 13 % (37/280) were not considered to be good LVAD candidates, 8 % (24/280) failed to keep their appointment, and 3 % (9/280) refused to accept our advice for LVAD or transplant. Only patients implanted as part of the HMII trial were studied since complete datasets were available only for these patients.
3.2.5 Outcomes
The two groups were compared with regard to preoperative patient characteristics and outcome measures including Kaplan–Meier survival , prevalence and incidence of adverse events , quality of life metrics (Kansas City Cardiomyopathy Questionnaire [KCCQ], clinical summary score [CSS], and overall summary scores [OSS], Minnesota Living With Heart Failure Questionnaire [MLWHF]), and functional status six-minute walk distance [6MWD], New York Heart Association (NYHA) function class, and patient activity levels with Metabolic Equivalent Task Score (METS) [21].
3.2.6 Statistical Analysis
Statistical analyses except for Poisson regression were done using Systat (Cranes Software, Chicago, IL). Poisson regression was performed using SAS (SAS Institute Inc., Cary, NC). Differences between groups of independent, normally distributed, continuous variables were evaluated using the t-test. Variables that were not normally distributed were evaluated using the Mann–Whitney U-test. Normality was checked using the Anderson–Darling test . Differences in categorical variables were evaluated using the Fisher’s exact test . Statistical comparisons were two-sided and the level of significance was set at p < 0.05. Survival analysis was performed using the Kaplan–Meier method with patients censored for transplantation , recovery of the native heart function with device removal, or withdrawal from the study. Comparison of survival between the two groups was performed using the log-rank test. Adverse events were presented as both percentages of patients and event rates (events per patient-year). Comparisons of adverse event rates between the two groups were performed using a Poisson regression model (Mantel–Haenszel), with the total duration of support as the exposure time and total number of events as the response variable. Quality of life comparisons were performed using linear mixed effects modeling (mixed subroutine in Systat). The predictor variables were age group and time group (baseline, 1, 3, and 6 months). Time group: Eight patients (four in each group) had a previous pulsatile flow LVAD which was replaced with a HMII LVAD. Duration of support and survival for these patients was evaluated from the date of the first LVAD implant. Only adverse events that were observed when on the HMII device were included.
3.3 Results
3.3.1 Baseline Patient Characteristics
The age distribution of LVAD patients in this study shows most who were between the ages of 60 and 80 (Fig. 3.1). Baseline characteristics were similar between groups except for age, nutritional status (prealbumin), use of ACE inhibitors, and ventilator support (Table 3.3). There was a tendency for a higher prevalence of ischemic etiologies and prior cardiac resynchronization therapy in the >70 age group compared to the <70 age group, but these differences were not statistically significant. All patients were in NYHA class IV prior to the LVAD implant. There was no difference in the mean Leitz–Miller destination therapy risk score [31] between the <70 (10.5 ± 6.3) and >70 (8.3 ± 5.8, p = 0.205) groups, nor in the percentage of patients with high/very high-risk scores or low-risk scores.
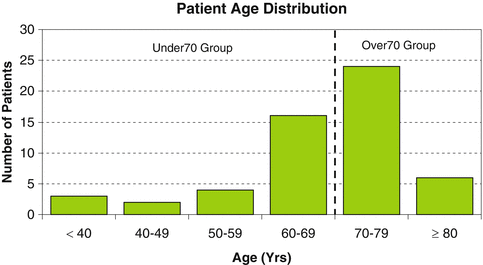

Fig. 3.1
Age distribution of patients evaluated in this study
Table 3.3
Baseline characteristics of patients used in the study. Body surface area (BSA), weight, cardiac index (CI), pulmonary capillary wedge pressure (PCWP), systolic blood pressure, albumin, prealbumin, and destination therapy risk score (DTRS) were normally distributed and evaluated using the t-test. The remaining continuous variables [left ventricular ejection fraction (LVEF) , creatinine, blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBILI), sodium (NA)] were evaluated using the nonparametric Mann–Whitney U-test
Parameter | Age group | P | |
|---|---|---|---|
Under 70 years (n = 25) | Over 70 years (n = 30) | ||
Patients enrolled (%) | 25 (45) | 30 (55) | – |
Age (years) [min–max] | 56.7 ± 14.3 [16–69] | 76.3 ± 3.9 [70–87] | <0.001 |
Ischemic (%) | 15 (60) | 24 (80) | 0.140 |
BSA (m2) | 1.98 ± 0.21 | 1.95 ± 0.19 | 0.671 |
Weight (kg) | 83 ± 15 | 79 ± 15 | 0.276 |
LVEF (%) | 21 ± 9 | 20 ± 6 | 0.651 |
CI (L/min/m2) | 1.95 ± 0.72 | 1.67 ± 0.49 | 0.139 |
PCWP (mmHg) | 27 ± 9 | 27 ± 9 | 0.824 |
Systolic BP (mmHg) | 104 ± 19 | 108 ± 15 | 0.438 |
Creatinine (mg/dL)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||