9 OBJECTIVES
Occupational/Inhalational/Environmental Disease
 Identify the lung diseases caused by inhalation of foreign material from the environment or during the course of specific occupations.
Identify the lung diseases caused by inhalation of foreign material from the environment or during the course of specific occupations.
 Identify the lung diseases related to adverse environmental conditions such as near drowning and ascent to high altitudes (mountain sickness).
Identify the lung diseases related to adverse environmental conditions such as near drowning and ascent to high altitudes (mountain sickness).
 Understand the pathophysiology of pulmonary reactions to these insults.
Understand the pathophysiology of pulmonary reactions to these insults.
 Describe the clinical presentation and treatment of the various disorders.
Describe the clinical presentation and treatment of the various disorders.
GENERAL CONSIDERATIONS: FACTORS AFFECTING DEPOSITION OF INHALED MATERIAL
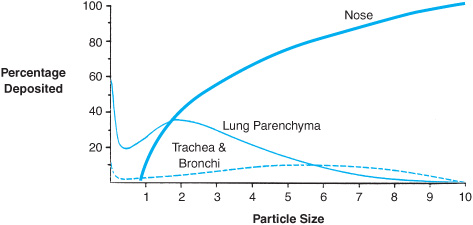
The site of deposition of material inhaled into the respiratory tract is dependent on water solubility for gases and particle size for solids. Highly soluble gases such as ammonia will be trapped in the moist mucus lining of the nose and oropharynx, while less soluble gases such as phosgene may cause damage to the large airways or the lung parenchyma. Particle size or aerodynamic diameter is of greatest importance in determining the level of deposition for inhaled particulates (Figure 9–1). Large particles (>10 μm) are efficiently filtered by the nose, although filtration efficiency decreases with exercise because of mouth breathing and increased airflow. Particles 3–10 μm in diameter are deposited preferentially in the trachea and large airways, while particles 0.1–3 μm in size reach the alveolar space. Very small particles (<0.1 μm) do not settle out from the air stream and are poorly deposited at any location in the respiratory tract, and many are exhaled. For inhaled fibers, deposition is based upon their aerodynamic diameter rather than length, which explains why very long asbestos fibers may penetrate deeply into lung tissue.
The likelihood that an inhaled gas or particle will cause pulmonary disease is dependent not just on deposition within the respiratory tract, but also on the total burden of inhaled material retained in the lungs over time. Tissue burden is not only influenced by particle size, but also by the concentration of material within the inhaled air stream, the duration of exposure, and the clearance mechanisms operative at various anatomic levels of the respiratory tract. Thus, both short-term but high-intensity and low-intensity but long-term exposures may cause pulmonary disease. Finally, host factors due to genetic differences or concomitant pulmonary disease such as emphysema significantly influence pulmonary reactions to inhaled materials.
Figure 9–1. Where an inhaled particle deposits within the respiratory tract is dependent upon the size of the particle. (Based on data from Bates DV, Fish BR, et al: Deposition and retention models for internal dosimetry of the human respiratory tract. Task group on lung dynamics. Health Phys. 1966;12:173.)
OCCUPATIONAL ASTHMA
Occupational asthma is defined as sensitivity to inhaled materials in the workplace with development of asthmatic symptoms de novo. It should be distinguished from exacerbation of underlying asthma in the workplace environment related to fumes, dusts, cold air, and the like. Occupational asthma requires both exposure to a specific antigen and individual susceptibility. The mechanisms for this susceptibility are poorly understood, but it is well recognized that one worker may develop asthma while many coworkers with identical exposures remain asymptomatic.
Pathogenesis
Occupational asthma can be divided into two categories depending on the molecular weight of the implicated antigen. High molecular weight antigens are often organic in nature. Workers who develop asthma in relation to these antigens are often atopic and presumably more susceptible to asthma. For example, animal care workers may become sensitized to animal proteins, and bakers may develop asthma in response to inhalation of flour. Low molecular weight antigens may also induce an asthmatic response, but there is often no underlying atopy. Examples of such antigens include plicatic acid which is contained in red cedar wood and toluene diisocyanate which is contained in some paints and chemicals.
Pathophysiology
 The end result of this sequence of events is airway obstruction and increased resistive work of breathing. Static lung compliance is unaltered, but airway obstruction reduces dynamic compliance. Thus, many alveoli have delayed emptying and may not empty fully at the end of exhalation. Retained air within these lung units will increase residual volume (RV) and functional residual capacity (FRC). Gas exchange is also compromised during an asthma attack, since mediator release interferes with hypoxic pulmonary vasoconstriction and poorly ventilated alveoli are perfused, causing hypoxemia. In severe asthma, the increased resistive work of breathing may precipitate acute respiratory failure with CO2 retention and respiratory acidosis. However, asthma is a reversible disease. With treatment or cessation of exposure to the antigen, all of these pathophysiologic processes may resolve completely.
The end result of this sequence of events is airway obstruction and increased resistive work of breathing. Static lung compliance is unaltered, but airway obstruction reduces dynamic compliance. Thus, many alveoli have delayed emptying and may not empty fully at the end of exhalation. Retained air within these lung units will increase residual volume (RV) and functional residual capacity (FRC). Gas exchange is also compromised during an asthma attack, since mediator release interferes with hypoxic pulmonary vasoconstriction and poorly ventilated alveoli are perfused, causing hypoxemia. In severe asthma, the increased resistive work of breathing may precipitate acute respiratory failure with CO2 retention and respiratory acidosis. However, asthma is a reversible disease. With treatment or cessation of exposure to the antigen, all of these pathophysiologic processes may resolve completely.
Diagnostic & Clinical Implications
SYMPTOMS & PHYSICAL FINDINGS
Because occupational asthma involves immunologic sensitization, there is often a delayed period from first exposure to the development of symptoms. This latent interval varies from weeks to years. Many workers with occupational asthma complain of chest tightness, wheezing, cough, and dyspnea within 10–20 minutes of exposure to a particular material. If they are examined immediately, it may be found that such workers have tachypnea and wheezing on auscultation of the chest. However, with removal from antigen exposure, the symptoms resolve and the findings on physical examination are often normal. Other workers may have a delayed or late onset of bronchoconstriction following exposure (see section on pulmonary function tests [PFTs] below). In these individuals, symptoms may not occur until 4–6 hours after exposure, which may make it difficult to relate the symptoms to a specific exposure.
IMAGING
Because asthma affects the airways and not the alveoli, the chest x-ray is usually normal. During an attack or with repeated attacks, hyperinflation may be visible on x-ray.
PULMONARY FUNCTION TESTS
Pulmonary function abnormalities in occupational asthma vary with time after antigen exposure. Twenty-four hours after exposure, pulmonary function may be normal. The hallmark of occupational asthma is a bronchoconstrictor response to the specific antigen implicated in the disease. This response can be duplicated in the laboratory with aerosolization of graded concentrations of the suspect antigen with repetitive pulmonary function testing. Such an inhalation challenge can be very helpful in identifying specific antigens responsible for symptoms so that work practices can be modified to decrease exposure. Patients with occupational asthma are hyperresponsive to specific antigens and show an exaggerated drop in forced expiratory volume in the first second (FEV1) and in the FEV1:FVC (forced vital capacity) ratio with the causal antigen, and no change in FEV1 with irrelevant antigens. Two patterns are seen in response to aerosolized antigen. Early or immediate-onset reactions may be seen within 10–20 minutes and last for about 2 hours. These reactions generally reflect IgE-mediated responses. In other patients, reactions are delayed (late onset) for 4–6 hours following exposure and may last for up to 24 hours. These reactions may reflect IgG-mediated responses. Combination, or dual-onset, responses are also seen.
Management Principles
Therapy for occupational asthma revolves around identifying the causal antigen and modifying work practices to eliminate further exposure. Bronchodilators and corticosteroids may help treat symptomatic workers but will eventually fail if antigen exposure continues.
LUNG DISEASE FOLLOWING INHALATION OF INORGANIC DUSTS: PNEUMOCONIOSES
 Pneumoconioses are fibrotic lung diseases of the pulmonary parenchyma following chronic inhalation of inorganic dusts, typically during work exposures such as mining. The three pneumoconioses discussed here are silicosis, coal worker’s pneumoconiosis (CWP), and asbestosis.
Pneumoconioses are fibrotic lung diseases of the pulmonary parenchyma following chronic inhalation of inorganic dusts, typically during work exposures such as mining. The three pneumoconioses discussed here are silicosis, coal worker’s pneumoconiosis (CWP), and asbestosis.
Silicosis follows the inhalation of airborne silica fibers. High-risk occupations include hard rock miner, stone cutter, foundry worker, sandblaster, and ceramic worker. Acute and accelerated forms of silicosis have been described, but most cases occur in workers with long-term exposure (more than 15 years).
CWP is caused by the inhalation of coal dust during mining or processing of coal. Similar conditions may occasionally be seen in graphite miners. Coal dust is not a uniform material but contains variable amounts of carbon and mineral contaminants, including clay and quartz. In many coal miners, lung disease is caused by quartz contained in the coal and is more accurately termed silicosis than CWP.
Asbestosis follows the inhalation of asbestos fibers. Asbestos is a group of fibrous silicates with widespread application in industry. High-risk occupations include asbestos miner, asbestos textile worker, sheet metal and insulation worker, boilermaker, and pipefitter.
Pathogenesis
Retention of inhaled inorganic particles stimulates fibrosis within the lung interstitium. Lung tissue from workers with silicosis shows fibrotic nodules preferentially involving the upper lobes. Microscopically, these nodules can be seen to contain silica fibers in the center surrounded by whorls of collagen and a cellular capsule containing fibroblasts. Silicotic nodules are initially localized around respiratory bronchioles and arterioles and in paraseptal and subpleural locations. With advanced disease, the surrounding fibrosis destroys lung tissue, and nodules may become confluent. Nodules may also be found in hilar lymph nodes, which often show peripheral, or “eggshell,” calcification. In CWP, the principal pathologic lesion is the coal macule, a pigmented lesion containing dust-laden macrophages surrounding respiratory bronchioles. In contrast to silicotic nodules, coal macules contain little collagen and there is no surrounding fibrosis. Lungs of workers with asbestosis show peribronchiolar fibrosis characteristically involving the lower lobes. Alveolar spaces may be filled with inflammatory cells, and asbestos bodies (asbestos fibers or other mineral particles such as glass, iron, or talc coated with hemosiderin, generically called ferruginous bodies) are usually seen with special stains.
Pathophysiology
The consequence of parenchymal fibrosis in silicosis and asbestosis is decreased lung compliance due to increased elastic recoil. Since affected individuals must therefore generate a greater transpulmonary pressure to inflate the lungs to the same volume, the work of breathing is increased, initially with exercise, and with advanced disease at rest. In asbestosis, although airways may be destroyed, pulmonary resistance is usually normal. In silicosis, involvement of small airways may contribute to airflow obstruction and emphysema. In CWP, the absence of fibrosis means that both lung compliance and resistance are normal unless there is concomitant silicosis or smoking-related obstructive disease.
Besides parenchymal fibrosis, inhaled asbestos fibers also migrate to reach the parietal pleura, where they commonly stimulate the formation of fibrous plaques with calcification. Extensive pleural plaques may cause increased tissue resistance (decreased chest wall compliance) and increased work of breathing. Pleural plaques are also seen to a lesser extent in silicosis. Pleural reactions are not seen in CWP.
Work exposures to both asbestos and silica are associated with increased risk of intrathoracic malignancy. By itself, CWP does not alter individual cancer risk. The signal neoplasm associated with asbestos exposure is malignant mesothelioma. This rare and usually fatal tumor may involve the pleural or peritoneal space. Approximately three quarters of patients with malignant mesothelioma have a significant work or environmental exposure to asbestos. Asbestos workers are also at increased risk for all types of lung cancer, particularly if they also smoke cigarettes. The increased risk of lung cancer appears to be restricted to those workers who have developed parenchymal fibrosis or asbestosis. Emerging epidemiologic evidence supports a similar relationship between silicosis and lung cancer.
Diagnostic & Clinical Implications
SYMPTOMS & PHYSICAL FINDINGS
Symptoms are caused by increased work of breathing as well as through stimulation of nerves in the interstitial space (J receptors). Exertional dyspnea is slowly progressive over many years. A productive or nonproductive cough may also be present. In CWP, symptoms usually reflect tobacco-related disease rather than the pneumoconiosis itself. Physical findings include inspiratory crackles on auscultation of the chest. Digital clubbing may be present in asbestosis but not in silicosis or CWP.
IMAGING
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree