Obstructive and Regurgitant Lesions
“One cannot know how to treat a disease until one understands the natural history of the disease”
Aortic Stenosis and Insufficiency
Incidence and Types
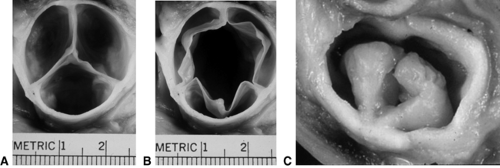
There are four types of aortic stenosis. The most common is aortic valve stenosis (see Fig. 11-1), which comprises approximately 5% of congenital heart defects. The aortic valve cusps or leaflets are congenitally malformed (Fig. 11.1); they may be thickened and the raphe may be fused. Usually, one of the cusps is quite underdeveloped, resulting in so-called bicuspid aortic valve. Indeed, about 1% to 2% of the general population may have a bicuspid aortic valve, but the valve may not be stenotic or insufficient.
The second type of aortic stenosis is supravalvar aortic stenosis. This involves a narrowing of the ascending aorta just above the sinotubular junction. This narrowing may be quite discreet or diffuse. Supravalvar stenosis can occur sporadically, but there is a familial form that results from a defect in the elastin gene on chromosome 7. Supravalvar stenosis also occurs in Williams syndrome, which is associated with typical facies, mental subnormality, and a defect in the elastin gene.
The third type of aortic stenosis is subvalvar aortic stenosis. In this condition, there is a fibromuscular ridge that causes obstruction a short distance below the valve. In addition, turbulence of blood flow caused by the obstruction can damage the valve leaflets, resulting in aortic valve insufficiency. Also, the leaflets of the valve can be fused to the membrane, which can cause leakage of the valve. If instead of a ridge, there is a longer area of obstructive tissue, it is called tunnel subaortic stenosis.
The fourth type of aortic stenosis is muscular subaortic stenosis. This is actually a type of hypertrophic cardiomyopathy and is referred to as hypertrophic obstructive cardiomyopathy or hypertrophic subaortic stenosis (see Chapter 13). This lesion is not discussed in this chapter but will be dealt with in the section dealing with cardiomyopathy.
Aortic insufficiency frequently occurs in conjunction with aortic valve stenosis, especially if the valve is bicuspid. However, aortic insufficiency can occur because of a bicuspid aortic valve even in the absence of aortic stenosis. Aortic insufficiency is frequently a complication of balloon valvuloplasty or surgical aortic valvotomy and may result from endocarditis on
a previously stenotic valve. Aortic insufficiency also is associated with tetralogy of Fallot, pulmonary atresia, Marfan syndrome, and other connective tissue disorders.
a previously stenotic valve. Aortic insufficiency also is associated with tetralogy of Fallot, pulmonary atresia, Marfan syndrome, and other connective tissue disorders.
Clinical Presentation
Aortic valve stenosis is more common in men than in women.
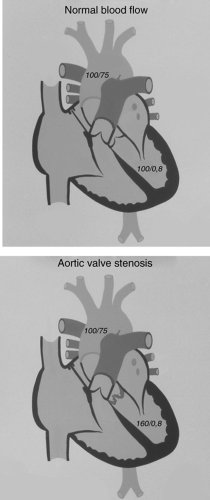
The clinical presentation of aortic valve stenosis depends on the severity of the obstruction. The measure of severity is based on the pressure drop across the valve during systole. Normally, when the left ventricle contracts, the pressure in the ventricle is the same as that in the aorta (see Fig. 11-2); that is, there is no pressure drop or gradient across the aortic valve. In trivial aortic stenosis, the pressure difference is 0 to 25 mm Hg, in mild stenosis it is 26 to 50 mm Hg, in moderate stenosis it is 51 to 79 mm Hg, and in severe stenosis it is >80 mm Hg. This classification of severity assumes that cardiac output is normal. If cardiac output is reduced, this classification is unreliable, a situation that may occur in infants with severe aortic stenosis.
Most patients with aortic stenosis are asymptomatic and are diagnosed because of the presence of a cardiac murmur. Rarely, syncope is the presenting sign of aortic stenosis. Some infants with severe aortic stenosis can present with low cardiac output, severely reduced ejection fraction, shock, and/or congestive heart failure. This is referred to as critical aortic stenosis. If an infant has poor left ventricular function and aortic stenosis (but without a very high transaortic gradient), aortic valve stenosis still is the likely cause of the poor left ventricular function and must be relieved.
Aortic insufficiency usually presents with a murmur. Severe or progressive aortic insufficiency may present with fatigue, shortness of breath, exercise intolerance, orthopnea, and/or paroxysmal nocturnal dyspnea.
Physical Examination
In children and adolescents with aortic stenosis, the peripheral pulses and pulse volumes are normal, and there is no cyanosis.
In patients with mild or moderately severe aortic stenosis, the precordial impulses may be normal. In patients with moderate or severe stenosis, the apical impulse may be increased. There may be a thrill palpable at the base of the heart and over the carotid arteries.
The first and the second heart sounds are normal. With aortic valve stenosis (but not with supravalvar or subvalvar stenosis), an ejection click will be heard immediately after the first
heart sound. This is best heard with the diaphragm of the stethoscope over the apex of the heart or the mid-left sternal border. It is a high-frequency sound. The absence of an aortic ejection click in the presence of other findings consistent with aortic stenosis suggests the presence of subvalvar or supravalvar aortic stenosis.
heart sound. This is best heard with the diaphragm of the stethoscope over the apex of the heart or the mid-left sternal border. It is a high-frequency sound. The absence of an aortic ejection click in the presence of other findings consistent with aortic stenosis suggests the presence of subvalvar or supravalvar aortic stenosis.
The murmur of aortic stenosis is systolic and crescendo–decrescendo or “diamond shaped.” It is best heard along the left sternal border and radiates to the right upper sternal border. The
correlation between the intensity of the murmur and severity of aortic stenosis is not good. However, it is unusual for a murmur of grade 2/6 or less to be associated with severe stenosis. Some patients with aortic stenosis have associated aortic insufficiency, in which case a decrescendo diastolic murmur will be audible.
correlation between the intensity of the murmur and severity of aortic stenosis is not good. However, it is unusual for a murmur of grade 2/6 or less to be associated with severe stenosis. Some patients with aortic stenosis have associated aortic insufficiency, in which case a decrescendo diastolic murmur will be audible.
Aortic insufficiency is characterized by a decrescendo murmur that begins immediately after S2. The pulse pressure is wide, resulting in “bounding” or “collapsing” pulses. Because there is left ventricular volume overload, the apical impulse may be prominent. Because aortic insufficiency frequently occurs with aortic valve stenosis, the clinical findings of aortic stenosis also may be present.
Electrocardiographic Features
Aortic stenosis and insufficiency are associated with electrocardiographic features of left ventricular hypertrophy (LVH), but the association is inconsistent. The correlation between the presence and/or absence of LVH on the electrocardiogram (ECG) and the severity of aortic stenosis or aortic insufficiency is not good. If there is flattening or inversion of the T waves in leads V5 and V6, then it is likely that the aortic stenosis is severe. With severe aortic insufficiency, there may be evidence of myocardial ischemia.
Chest x-ray
With aortic stenosis, the heart size usually is normal. Because poststenotic dilation of the aorta occurs with aortic stenosis, the ascending aorta may appear prominent on the chest x-ray. The chest x-ray is not particularly helpful in determining the severity of aortic stenosis.
In contrast to aortic stenosis, the chest x-ray is helpful in determining the severity of aortic insufficiency. Increasing cardiomegaly suggests progressive aortic insufficiency and is helpful in deciding the timing of operation.
Echocardiography and Cardiac Catheterization
The severity of aortic stenosis is based on the pressure gradient across the abnormal aortic valve. Historically, these gradients were measured directly at the time of cardiac catheterization. Indeed, before the era of echocardiography, essentially all patients with aortic stenosis had cardiac catheterization to confirm the diagnosis and to determine the severity of aortic stenosis. Using echocardiography/Doppler techniques it is now possible, noninvasively, to estimate the transaortic pressure gradient. One must keep in mind, however, that this is only an estimate. The Doppler assessment of aortic stenosis will provide maximum instantaneous, as well as a mean, transaortic gradient. Most people think that the peak-to-peak gradient that is measured at the time of cardiac catheterization is somewhere between the mean and maximum instantaneous Doppler gradient. Using echocardiography, one can assess left ventricular wall thickness, which also is a reflection of severity.
Echocardiography and Doppler can confirm the presence of aortic insufficiency and are useful in determining the severity of the leakage. Using these techniques, one can determine the regurgitant fraction, the left ventricular systolic and diastolic dimensions, and the left ventricular ejection fraction.
Treatment
Treatment of aortic valve stenosis involves manipulating the valve to reduce the stenosis. This can be accomplished by transvenous balloon dilation of the valve, open surgical
valvotomy, or surgical valve replacement. In the absence of significant aortic regurgitation, most cardiologists favor transvenous balloon dilation of the valve as the initial procedure for infants and young children. If there is aortic stenosis and significant aortic regurgitation, open surgical valvotomy and valve repair or replacement is the preferred treatment.
valvotomy, or surgical valve replacement. In the absence of significant aortic regurgitation, most cardiologists favor transvenous balloon dilation of the valve as the initial procedure for infants and young children. If there is aortic stenosis and significant aortic regurgitation, open surgical valvotomy and valve repair or replacement is the preferred treatment.
Valve replacement includes several possibilities. The stenotic valve could be replaced with a homograft, porcine, or mechanical valve. The advantage of a homograft or porcine valve is that the patient does not have to take warfarin and hence avoids the potential complications of anticoagulation. The disadvantage of these tissue valves is that they last only 8 to 10 years and must then be replaced. The advantage of a mechanical valve is its superior longevity, but lifelong anticoagulation is necessary.
An additional option is the Ross procedure. In this operation, the patient’s pulmonary valve is removed and sewn into the aorta in place of the stenotic aortic valve. A homograft valve is then used in the pulmonary position. Unfortunately, after the Ross procedure, the neoaortic valve and the homograft placed into the pulmonary position may fail.
Patients with mild or moderate stenosis do not need balloon dilation or operation. It is generally agreed that patients with transaortic gradient >80 mm Hg should undergo the balloon dilation or operation to reduce the degree of stenosis. In patients with gradients between 50 and 80 mm Hg, the decision to relieve the stenosis is made after considering a number of factors including the patient’s age, the presence of associated aortic insufficiency, and left ventricular wall thickness. There are two situations in which the decision to relieve the stenosis might not be related to the magnitude of the gradient. The first is the case of an infant with critical aortic stenosis and congestive heart failure and/or low cardiac output. Relief of the stenosis is indicated in this situation regardless of the magnitude of the gradient. The second situation is that of a patient with syncope or symptoms that clearly are related to the aortic stenosis.
In the Second Natural History Study of Congenital Heart Defects, the 25-year survival of patients with aortic stenosis was 85.1% as compared to an expected survival of 96.4%. In patients with trivial or mild stenosis, the 25-year survival was 92.4% as compared to 81% for patients with moderate or severe stenosis. The following were associated with poorer survival: Male sex, higher transaortic gradient, cardiac symptoms, and older age. In patients who had surgical aortic valvotomy, there was a 40% chance that a second operation would be necessary over a 25-year period. The incidence of bacterial endocarditis was 27.1 per 10,000 person-years of follow-up.
There are several approaches to the management of the patient with significant aortic insufficiency. The options for valve replacement are the same as those discussed in the preceding text for aortic stenosis. In addition, the surgeon can, in select cases, repair the valve. Unfortunately, repair of an insufficient valve fails relatively early in at least 10% of cases. The long-term outcome of aortic insufficiency depends on the severity of the insufficiency and the type of procedure employed to deal with the leakage.
The treatment of discreet or tunnel subvalvar aortic stenosis is surgical resection of the obstructing tissue. At times, it is necessary for the surgeon to perform a “modified Konno” procedure to enlarge the left ventricular outflow tract. This involves creating a ventricular septal defect (VSD) in the outlet septum just below the aortic valve and then closing this defect with a patch, such that the outflow tract is larger. In the absence of aortic insufficiency, the indications for operation are similar to those for aortic valve stenosis. However, if there is significant aortic valve insufficiency, one would operate for a lower transaortic pressure gradient because discreet subvalvar aortic stenosis can cause progressive aortic valve insufficiency, presumably owing to injury to the valve leaflets caused by the
eccentric jet of blood through the subaortic area. In addition, the membrane may be attached to the valve leaflets, contributing to the aortic valve insufficiency.
eccentric jet of blood through the subaortic area. In addition, the membrane may be attached to the valve leaflets, contributing to the aortic valve insufficiency.
The treatment of supravalvar aortic stenosis involves patch enlargement of the narrow area of the aorta. The indications for operation are the same as those for aortic valve stenosis.
All patients with any type of aortic stenosis or insufficiency must observe prophylactic measures for infective endocarditis.
Coarctation of the Aorta
Incidence and Associated Anomalies
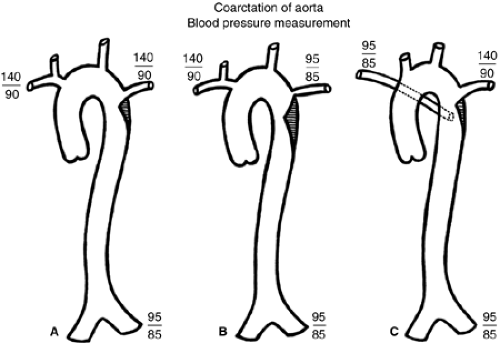
Coarctation of the aorta constitutes 5% of congenital cardiac defects. It consists of a ledge-shaped protrusion of tissue into the aorta at a position immediately opposite the insertion of the ductus or ligamentum arteriosus (see Fig. 11.3 and 11.4). In 50% to 85% of cases, there is an associated bicuspid aortic valve. Less commonly, it can be associated with a VSD or subvalvar aortic stenosis. If coarctation is associated with subaortic stenosis and a parachute mitral valve and supravalvar mitral membrane, the complex is referred to as Shone syndrome.
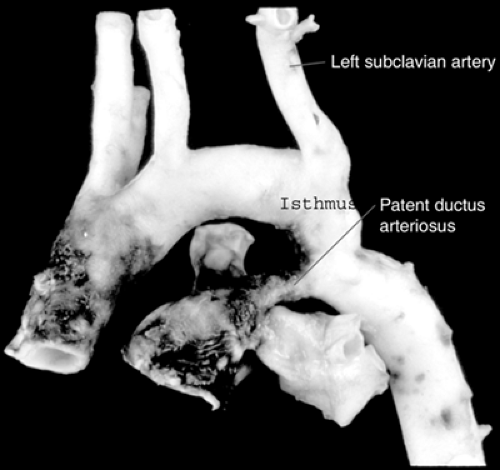 Figure 11.4 • Pathologic specimen of coarctation of the aorta. Note that the coarctation is immediately opposite the site of the insertion of the ductus arteriosus.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|