Compounds
Adverse clinical cardiorenal outcomes
Cardiac effects
Vascular effects
Renal effects
Indoxyl sulfate
Increased collagen synthesis in NCF [34]
Defective endothelial proliferation and wound repair in vitro [35]
CV mortality [27]
Increased protein synthesis in NCM [34]
Enhanced oxidative stress determined by an increase in ROS production and NADPH oxidase activity, and a reduction in glutathione levels in cultured HUVEC [39]
All-cause mortality [27]
Diastolic LV dysfunction [42]
Promote ROS production and a senescence in cultured HUVEC [43]
Promote VSMC proliferation in vitro [47]
Increased cardiac oxidative stress in vivo [52]
Promote aortic calcification and cell senescence in vivo in association with an increased expression of senescence-related proteins such as p16 (INK4a), p21(WAF1/CIP1), p53 and retinoblastoma protein [53]
Renal fibrosis in association with CpG hypermethylation of the Klotho gene (a renoprotective antiaging gene) and decreased Klotho expression in renal tubular cells both in vitro and in vivo [46]
Promotes ROS generation and osteoblastic transformation of aortic smooth muscle cell in vitro by increasing expression of osteoblast-specific proteins such as core binding factor 1, osteopontin and alkaline phosphatase [54]
Promote cell senescence in the kidneys by down-regulating renal klotho gene and protein expression both in vitro and in vivo, in association with ROS production and activation of nuclear factor-kB in renal proximal tubular cells [55]
Enhance leukocyte adhesion and extravasation and interrupt blood flow [56]
p-cresyl sulfate
Increased collagen synthesis in NCF [34]
Promote endothelial dysfunction by inducing Rho kinase-mediated microparticle release from cultured HUVEC [57]
Increased inflammatory gene expression in cultured renal proximal tubular cells [40]
Increased protein synthesis in NCM [34]
Increased endothelial permeability to albumin in vitro, in the presence of p-cresyl glucoronide [58]
Glomerulosclerosis and renal interstitial fibrosis with activation of pro-fibrotic gene and protein expression in vivo [45]
Enhance leukocyte rolling in vivo [56]
Renal fibrosis in association with CpG hypermethylation of the Klotho gene (renoprotective antiaging gene) and decreased Klotho expression in renal tubular cells both in vitro and in vivo [46]
Impaired blood flow and cause vascular leakage, in the presence of p-cresyl glucoronide in vivo [56]
p-cresol (present in the body as its conjugated forms, mainly p-cresyl sulfate)
CV events [31]
Increased protein synthesis in NCM [34]
Decreased endothelial proliferation and wound repair in vitro [35]
Potentially inducing renal tubular adenoma [59]
All-cause mortality [30]
Abnormal changes in the gap junction in cultured cardiomyocytes [60]
Inhibit cytokine-induced endothelial adhesion molecule expression and endothelium/monocyte adhesion in vitro [61]
Phenylacetic acid
n/a
Increased protein synthesis in NCM [34]
n/a
Inducing inflammatory cytokine gene expression in vitro [62]
Indole-3-acetic acid
n/a
n/a
Induce CD133+ cell apoptosis in vitro [63]
Functional impairment [64]
Glomerular sclerosis and interstitial fibrosis [64]
Enhancing renal oxidative stress in vitro [48]
Homocysteine
n/a (despite a strong association between homocysteine and poor cardiovascular outcomes demonstrated)
Oxidative stress-induced endothelial dysfunction and damage [67]
n/a
Increased expression of vascular inflammatory and thrombogenic mediators [68]
MAPK-mediated VSMC proliferation [69]
Promote calcium deposition and osteogenic differentiation in VSMCs cocultured with THP-1 cells (human leukemia monocytic cell line) [70]
Hippuric acid
n/a
n/a
n/a
Functional impairment [64]
Glomerular sclerosis [64]
Phenol
n/a
suppress contractility of cardiac muscle in vitro [71]
n/a
n/a
Hydroquinone
n/a
n/a
n/a
Tumorigenesis of renal tubules in animal models [72]
Interestingly, adverse effects of homocysteine can be worsened in the state of folate and vitamins B6 and B12 deficiency [68]. However, putative beneficial effects of folate and vitamin B supplementation on CV outcomes remain controversial. Results from large clinical trials vary from beneficial [75], neutral [76, 77] to even harmful CV effects [78].
Non-atherosclerotic Vascular Disease
PBUTs are implicated in vascular stiffness, calcification and ossification, common CKD-associated vascular abnormalities. Serum IS levels have been demonstrated to correlate with vascular stiffness [27], and both IS and pCS circulating levels correlate with vascular/aortic calcification in various stages of CKD [27, 29]. Pre-clinical studies (Table 19.1) have shown that IS [53] and homocysteine [70] promote vascular calcification in association with activation of cell senescence [53]. Both toxins have been demonstrated in vitro to be involved in osteogenic differentiation of vascular smooth muscle cell [54, 70].
Cardiac Remodeling and Dysfunction
Study of direct cardiac effects of PBUTs has been extremely rare. It is surprising that investigation of homocysteine on the heart or cardiac cells has never been reported despite its well-known adverse CV outcomes. Recently, pro-fibrotic and pro-hypertrophic effects of IS, cresol and cresol conjugates were investigated in cultured cardiac fibroblasts and myocytes (Table 19.1) [73]. Among the tested compounds, IS has strongest pro-fibrotic and pro-hypertrophic effects followed by pCS whilst p-cresol, m-cresol, m-cresyl sulfate and phenylacetic acid had little or no effect. IS also enhances gene expression of pro-inflammatory cytokines interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α in THP-1 cells [34]. The pro-fibrotic, pro-hypertrophic and pro-inflammatory effects of IS are likely mediated via activation of p38 mitogen-activated protein kinase (MAPK), p44/42 MAPK and nuclear factor-kappa B (NF-κB). This suggests that IS may be implicated in adverse cardiac remodeling processes. The follow-on in vitro study shows indirect evidence of intracellular IS uptake into cardiac myocytes and fibroblasts via organic anion transporters (OATs) 1 and 3 [79]. However, further investigation of cellular entry mechanisms of IS may be clinically useful since several OAT inhibitors are readily available and currently used for other indications.
Adverse Renal Effects
High serum pCS [26, 29] and IS [26, 27] levels are independently associated with progression of CKD in patients at different stages of CKD.
Most PBUTs are normally excreted in urine by renal tubular cells therefore these cells are likely to be the first injured by accumulated toxins. Among PBUTs, renal toxicity due to IS appears to be strongest, demonstrated both in vitro and in vivo (Table 19.1) [80–82]. Collectively, IS accelerates the progression of CKD due largely to its pro-fibrotic, pro-inflammatory and oxidative stress-inducing effects. Findings in favor of an upregulation of transforming growth factor-β1 (TGF-β1) and reactive oxygen species (ROS) have been associated with activation of NF-κB [48]. In addition, IS has been recently demonstrated to be implicated in renal cell senescence by reducing expression of an anti-aging gene namely klotho through increased production of ROS and activation of NF-κB in renal proximal tubular cells [55]. Thus, the ROS/NF-κB/TGF-β1 pathway is most likely involved in IS-induced renal toxicity.
Novel Treatments Targeting Protein-Bound Uremic Toxins
As previously mentioned, current treatment for ESRD patients is largely based on dialysis. Developments in dialysis technology to remove a whole range of retained uremic solutes has gradually progressed in the past decade but residual uremic symptoms still persist and additional survival years (as achieved with transplantation) are not observed. Increased circulating levels of PBUTs in the setting of CKD are generally explained by impaired renal excretory function. In fact, a number of PBUTs including IS and pCS are derived from the colon via microbiotic metabolism. Indoles and phenols, the prototypes of which are IS and pCS, are important colon-derived microbiotic compounds originating from dietary tryptophan and tyrosine, respectively. Thus, decreasing circulating levels of colon-derived PBUTs in CKD may be achieved by two means; enhancing PBUTs removal by new dialysis technology and suppressing colonic production (Fig. 19.1).
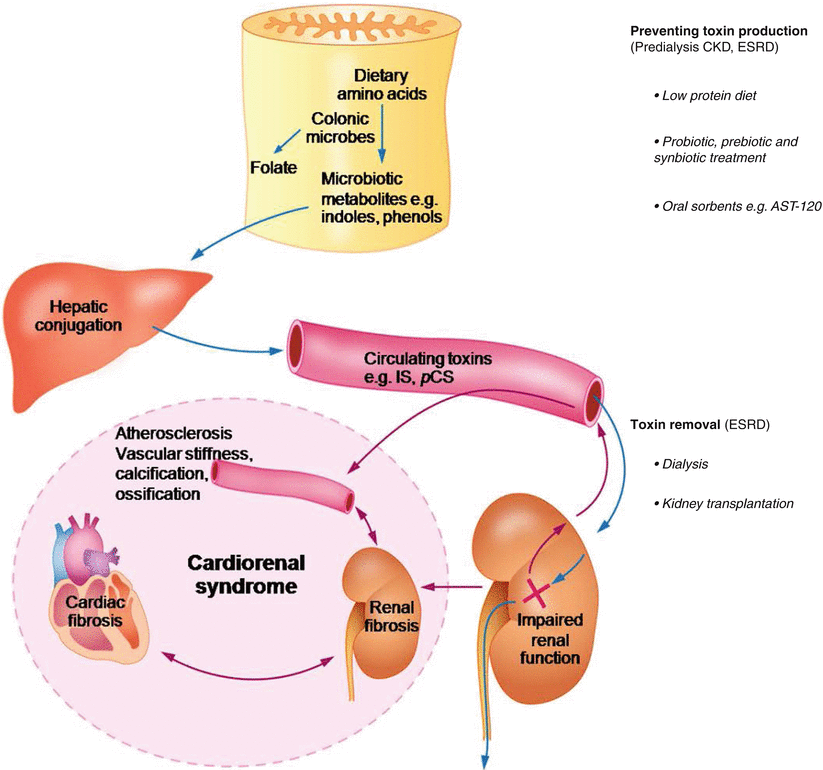

Fig. 19.1
Therapeutic strategies targeting colon-derived protein bound uremic toxins for cardiorenal syndrome. CKD chronic kidney disease, ESRD end-stage renal disease, IS indoxyl sulfate, pCS p-cresyl sulfate
Enhancing Removal by Dialysis Strategies
The efficacy of dialysis removal generally depends on the permeability and surface area/material of dialysis membrane, frequency of dialysis and time spent on each dialysis session [83, 84]. Removal of middle molecules by conventional dialysis has also been a problem but developments in dialysis technology and protocols have improved their clearance [22, 85]. For instance, compared to thrice a week by conventional HD, nocturnal HD 6 times a week significantly improves clearance of parathyroid hormone, a middle molecule, that is associated with an improvement in LVH [86]. However, the removal efficacy of most PBUTs is not significantly increased with more frequent dialysis, a larger dialyzer pore size (except hippuric acid) [87] or a convective strategy [85, 88]. At present, IS, pCS and homocysteine, all of which circulating levels are increased in CKD patients, appear to be most potentially toxic to the CV system. However homocysteine has been demonstrated to be better removed by nocturnal HD compared with a standard thrice-weekly HD [89], by high-flux HD compared with low flux HD [90], and use of convective dialysis [91]. There is currently no approved system for long-term dialysis-dependent ESRD patients to substantially improve removal of circulating IS and pCS. Use of protein-leaking membranes offers a better clearance of protein-bound solutes however large amounts (2–6 g/4 h) of albumin loss [92] could worsen the state of protein malnutrition usually present in dialysis patients.
Addition of sorbent system to conventional dialysis has been developed to improve removal of uremic solutes with a large MW or high protein binding capacity. A preliminary study showed promising results for clearance of tested middle molecules and cytokines with a MW range from 12 to 21 kD [93]. Another study using coated carbon hemoperfusion reported a limited removal of protein-bound solutes including IS due largely to the inadequate speed of carbon to take up solutes at low concentration, not the adsorptive capacity [94]. Recently, a dual layer hollow fiber mixed matrix membrane with embedded adsorptive carbon particles has been demonstrated to effectively remove the daily production of IS, pCS and hippuric acid [95].
The optimal sorbent system might augment the efficacy of conventional dialysis in PBUT removal thereby potentially providing clinical benefits, although invention is a big task in terms of its complexity and cost.
Treatment Suppressing Production of Colon-Derived Solutes
Changes in gut microbiome occur in the setting of CKD [96]. Increased amounts of protein delivered to the colon due to impaired intestinal absorption in CKD results in (1) increasing substrates for uremic solute production and (2) a shift of normal colonic bacterial fermentation pattern from a saccharolytic to proteolytic pattern [97]. The increased production of uremic solutes may be further accentuated by a prolonged colonic transit time commonly observed in long-term HD patients [98]. Decreasing colon-derived PBUTs by targeting the colon may be achieved by several means which are much simpler, safer and cheaper than dialysis treatment and could start at the early pre-dialysis stages of CKD.
Probiotic, Prebiotic and Synbiotic Treatment
Use of organisms lactic acid bacilli [99] and Bifidobacterium longum [100, 101] to restore the disturbed gut microbiome milieu or ‘probiotics’ reduces serum levels of IS in CKD patients on HD. The oral administration of Bifidobacterium longum in a gastroresistant seamless capsule to HD patients is also effective in reducing serum homocysteine levels [101], likely associated with a folate and vitamin B12 producing effect of probiotics. A small prospective, double-blind randomized-controlled crossover trial on probiotic treatment for 6 months in 46 patients with CKD stages 3 and 4 demonstrated a significant decrease in blood urea nitrogen and creatinine levels and an overall improvement in quality of life [102].
‘Prebiotics’, in contrast, uses a non-digestible food ingredient in order to selectively stimulate the activity of some colonic bacteria. Use of non-starch polysaccharides or digestion-resistant starches, considered as dietary fiber, could reverse the ratio of saccharolytic to proteolytic colonic microbial activity back to the normal state thereby limiting production of colon-derived solutes originating from dietary proteins. A non-randomized phase I/II study in maintenance HD patients demonstrated that administration of prebiotic oligofructose-enriched inulin for 4 weeks significantly reduces generation and serum levels of pCS but not IS [103].
‘Synbiotics’ is a combination of probiotic and prebiotic treatment. Synbiotic treatment, using Lactobacillus casei and Bifidobacterium breve as probiotics and galacto-oligosaccharides as prebiotics, for 2 weeks significantly decrease serum p-cresol levels in association with an improvement of bowel habits in HD patients [104].
Collectively, probiotic, prebiotic and synbiotic treatments show favorable effects on reducing problematic PBUTs, preserving renal function and improving quality of life. However, the impact of such treatments on major clinical endpoints such as CV events and mortality has not been studied.
Protein Restriction Diet
Protein restriction diet reduces substrates used in the production of colon-derived solutes. A very low protein diet has been demonstrated to significantly lower serum IS levels in predialysis CKD patients [105]. In this study a mixture of ketoanalogue and amino acid supplements was simultaneously administrated to prevent a negative nitrogen balance. In addition, any means that decrease colonic transit time such as laxatives could help suppress the production of colon-derived uremic solutes. Similar to probiotic and prebiotic treatment, study on major renal and cardiovascular outcomes of protein restriction diet is needed to clarify its potential role.
Oral Sorbents
Activated carbon has been widely used to remove various toxins from the gastrointestinal tract. AST-120 is a novel microspherical carbon adsorbent with high porosity and adsorptive capacity selective to low MW molecules (<10 kDa) [106] which therefore does not disturb GI enzymes (high MV) [107] or nutritional status. The kinetic profile of AST-120 is optimized for maximum binding in the lower GI tract where organic compounds including indoles and phenols, are produced. AST-120 is not degraded by digestive enzymes and intestinal bacteria thereby stimulating gastrointestinal excretion of such compounds after being adsorbed [107].
AST-120 effectively adsorbs colonic microbial metabolites and decrease circulating levels of uremic solutes in the setting of CKD such as IS, pCS, hippuric acid, phenyl sulfate, 4-ethylphenyl sulfate [108] and advanced glycation end-products [109]. AST-120 does not disturb creatinine and urea nitrogen balance therefore both traditional markers are acceptable for assessing renal function following AST-120 treatment [110].
Preclinical Studies
Renal Endpoints
AST-120 administration in uremic rats significantly reduces serum, renal and urinary IS levels [38, 107, 111] in association with an improvement in renal function and IS-induced renal tubulointerstitial fibrosis and glomerular sclerosis [36, 44, 107, 111–113]. Renal macrophage infiltration observed in failing kidneys is also suppressed by AST-120 [44].
Molecular study demonstrates that AST-120 decreases renal expression of pro-fibrotic (TGF-β1, tissue inhibitor of metalloproteinase-1 and pro-α1 (I) collagen), pro-inflammatory (intercellular adhesion molecule-1, osteopontin, monocyte chemotactic protein-1) and apoptosis-related (clusterin and osteopontin) genes [38, 107]. An antioxidative effect of AST-120 has been demonstrated in uremic rats [52, 112]. Anti-inflammatory, anti-fibrotic and anti-oxidative effects of AST-120 have been associated with attenuation of renal cortical NF-κB activation [44, 48].
Cardiac Endpoints
Administration of AST-120 in a severe CKD model with high serum IS levels significantly reduces cardiac fibrosis, TGF-β protein expression and NF-κB phosphorylation [42]. Interestingly, a reduction of serum IS levels with AST-120 treatment was positively correlated with extent of cardiac fibrosis. Another study in the same CKD model demonstrated a similar suppressing effect of AST-120 on cardiac fibrosis with a reduction in expression of cardiac oxidative stress markers, 8-hydroxydeoxyguanosine and acrolein [52]. These data suggests that IS-induced cardiac fibrosis may be potentially mediated via the ROS/NF-κB/TGF-β1 pathway similar to the proposed mechanistic pathway of IS-induced renal fibrosis [81].
Vascular Endpoints
AST-120 has been demonstrated to alleviate atherosclerosis by limiting plaque extension, inflammation and necrosis in a mice CKD model with apolipoprotein E-deficiency [115]. AST-120 also reduces hypercholesterolemia and plasma very low density lipoprotein in association with improving plasma lipoprotein lipase and hepatic lipase activity and increasing protein expression of lipoprotein lipase and very low density lipoprotein receptor in skeletal muscle and adipose tissue [113].
Clinical Studies
Treatment with AST-120 (>24 months) in pre-dialysis stage CKD patients is associated with a 3.5-fold reduced risk for initiation of dialysis [116]. For HD patients starting AST-120 treatment at the predialysis stage (average treatment duration 15.1 months), 5-year survival significantly improved compared with those with no treatment [117]. AST-120 treatment preserves renal function in diabetic patients with CKD, both early [118] and advanced stage [119]. Benefits on survival and cost reduction are also observed in advance stage diabetic nephropathy [120].
Improved renal function and delayed CKD progression by AST-120 have been associated with a decrease in serum and urinary IS levels [121] and plasma TGF-β1 levels [122]. An improvement of renal function over time was observed after ≥1-year suggesting benefit of long-term AST-120 treatment in pre-dialysis stage CKD patients [123].
Beneficial cardiovascular effects of AST-120 have been reported in CKD patients. AST-120 administration for 2 years (but not at 1 year) in predialysis patients significantly reduces carotid intimal media thickness and pulse wave velocity, a surrogate of vascular stiffness [124]. An improved aortic calcification index is observed in predialysis patients with 6-month duration of treatment [125]. AST-120 treatment for a relatively short treatment period of 6 months shows an improvement in IS-induced endothelial dysfunction by increasing flow-mediated endothelium-dependent vasodilatation in association with a reduction in IS levels and oxidative stress indicated by a decreased oxidized/reduced glutathione ratio [43]. Interestingly, predialysis CKD patients who are on AST-120 for at least 6 months have a significantly lower percentage of left ventricular concentric change compared with the untreated control group with comparable body mass index, systolic blood pressure, pulse pressure, history of coronary artery disease and prescribed medications [126], thereby potentially preventing LVH progression.
Nutritional status is improved with AST-120 by decreasing the free to protein-bound proportion of tryptophan leading to a less serotonin (an appetite suppressor) production and by increasing albumin and transferrin levels [127]. Combining AST-120 with a mild low protein diet shows a comparable effect on preserving renal function in CKD patients to a strict low protein diet [128]. Similarly, a prospective randomized controlled study has demonstrated that combined AST-120 and low protein diet is superior to a low protein diet alone [129].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


