Nontuberculous Mycobacterial Pulmonary Disease
Nontuberculous mycobacteria (NTM) are mycobacteria other than Mycobacterium tuberculosis and Mycobacterium leprae. Previous names for this group of organisms included “environmental mycobacteria,” “atypical mycobacteria,” or “mycobacteria other than tuberculosis” (1,2). Unlike M. tuberculosis, which is an obligate human pathogen with no environmental reservoir, NTM are commonly isolated from environmental sources such as water and soil (3,4). An increasing number of NTM have been recognized to be affecting the lung. Although the incidence of disseminated Mycobacterium avium complex (MAC) infections in patients with human immunodeficiency virus (HIV) has decreased in recent years with the use of highly active antiretroviral treatments (HAART), the rate of pulmonary NTM infection in other immunocompromised and nonimmunocompromised patients is increasing (5).
NTM have been traditionally classified into four groups on the basis of growth rates, colony morphology, and pigmentation (Runyon Classification System) (6). Groups I, II, and III are slow growers, requiring a time similar to that required by M. tuberculosis to grow in culture, whereas Group IV organisms are rapid growers that grow well in routine bacteriologic media in <7 days. The slow growers are further differentiated according to their ability to produce yellow pigment (3) (see Table 4.1).
The Runyon classification system has been primarily a tool for microbiologists, and has allowed easier identification of individual NTM species by mycobacterial laboratories. However, it has become less relevant in recent years because of advances in mycobacteriology, including more rapid culture techniques, DNA probes, and high-pressure liquid chromatography. In addition, this system is of little value to clinicians because the organisms in a particular Runyon class may cause different patterns of disease. A more appropriate grouping for these organisms is currently based on the type of clinical disease they produce: Pulmonary disease, lymphadenopathy, cutaneous disease, and disseminated disease (2,3).
Epidemiology
Most NTM are environmentally ubiquitous and have been recovered from water and soil. It is generally accepted that most human infection is due to environmental NTM (2,3). Person-to-person transmission of infection is rare and isolation of infected individuals is therefore not required. Although contact with environmental mycobacteria is
common, overt disease is uncommon because of the low virulence of these organisms. Disease usually develops in immunocompromised patients, in patients with preexisting lung disease, and only occasionally in otherwise apparently healthy persons (5).
common, overt disease is uncommon because of the low virulence of these organisms. Disease usually develops in immunocompromised patients, in patients with preexisting lung disease, and only occasionally in otherwise apparently healthy persons (5).
TABLE 4.1 Classification of Mycobacterial Species Commonly Causing Human Disease | |
|---|---|
|
There is considerable geographic variability in the prevalence of NTM disease and in the mycobacterial species responsible for it. Overall, the most common NTM resulting in pulmonary disease is the MAC (5). The second most common NTM pathogen is Mycobacterium kansasii in the United States and Japan and Mycobacterium xenopi in Canada and Europe, except for Scandinavia and areas of northern Europe, where Mycobacterium malmoense is second to MAC (7,8).
Diagnostic Criteria
Unlike M. tuberculosis, NTM are not obligate pathogens. Accordingly, the isolation of an NTM species from a respiratory sample is not sufficient evidence for the presence of NTM lung disease. The diagnosis of pulmonary NTM disease is based on clinical, radiographic, and bacteriologic criteria (1,2,3). The necessary clinical criterion is the presence of compatible symptoms and signs, with the reasonable exclusion of other etiologies of pulmonary disease. However, the signs and symptoms of NTM lung disease are variable and nonspecific. Clinical manifestations include chronic cough, fever, chills, night sweats, dyspnea on exertion, hemoptysis, and weight loss (5). NTM infection of the lungs often occurs in the context of preexisting lung disease, especially chronic obstructive pulmonary disease, bronchiectasis, pneumoconiosis, and previous tuberculosis. As a result, the clinical manifestations of NTM lung disease are often similar to those of the underlying disease (3).
The radiographic criteria required for diagnosis are the presence of consolidation, cavitation, or multiple nodules at plain chest radiography or high-resolution computed tomography (CT). The radiographic manifestations are variable, depending on the presence or absence of underlying disease, and on the NTM species (see Table 4.2).
In 1997, the American Thoracic Society issued a revised statement of diagnostic criteria for NTM lung disease (1,2) (see Table 4.3), and in 2000, the British Thoracic Society
published guidelines for the management of NTM disease (2). According to the British guidelines, which has less strict diagnostic criteria than those of the American Thoracic Society statement, NTM pulmonary disease is diagnosed when positive cultures develop from specimens of sputum obtained at least 7 days apart (two separate positive cultures) from a patient whose chest radiograph suggests mycobacterial infection and who may or may not have clinical symptoms or signs.
published guidelines for the management of NTM disease (2). According to the British guidelines, which has less strict diagnostic criteria than those of the American Thoracic Society statement, NTM pulmonary disease is diagnosed when positive cultures develop from specimens of sputum obtained at least 7 days apart (two separate positive cultures) from a patient whose chest radiograph suggests mycobacterial infection and who may or may not have clinical symptoms or signs.
TABLE 4.2 Radiologic Findings of Nontuberculous Mycobacterial Pulmonary Disease | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
TABLE 4.3 American Thoracic Society Criteria for the Diagnosis of Nontuberculous Mycobacterial Pulmonary Disease | ||||||
|---|---|---|---|---|---|---|
|
The American Thoracic Society diagnostic criteria put greater emphasis on multiple cultures using at least three sputum samples, the use of bronchoscopy with bronchial washing, transbronchial lung biopsy, and high-resolution CT scan, especially in patients without cavitation. These criteria were considered by some investigators as being primarily designed for use in the United States, where the incidence of tuberculosis is low and the relative incidence of NTM pulmonary disease is high (9). In developing countries, where the incidence of pulmonary tuberculosis is much higher than that of NTM pulmonary disease, the initiation of presumptive antituberculous treatment, especially in smear-positive patients prior to identification of isolates, is common practice (9). With empirical first-line antituberculous treatment, early sputum conversion to culture negativity would be expected in some cases of NTM pulmonary disease, reducing the likelihood of further positive culture of isolates. In general, evidence of disease, such as consistent pulmonary opacities on chest radiographs and the repeated isolation of multiple colonies of the same strain of NTM in the absence of other pathogens, is sufficient for the diagnosis of NTM pulmonary disease.
Because NTM pulmonary disease can be indolent, appropriate follow-up is essential to determine the significance of potentially pathogenic NTM isolated from sputum. Delays in diagnosis are frequent, and radiographs may remain unchanged for years. In one study, there was an average interval of 6.4 years before radiographic change was apparent (10). When NTM cultures are positive, stable findings at chest radiographs, especially at relatively short intervals, are not sufficient grounds to exclude infection. In the absence of lung biopsy, months to years of clinical, radiographic, and microbiologic follow-up of certain patients may be required to reliably determine the significance of NTM respiratory isolates (3).
Laboratory Methods
The methods of acid-fast staining and culture currently used for M. tuberculosis are acceptable for most NTM species. The appearance of NTM at microscopy is generally indistinguishable from that of M. tuberculosis, and the American Thoracic Society has recommended that samples should be inoculated onto at least one solid medium (Lowenstein–Jensen or Middlebrook 7H10 and 7H11) and into a liquid culture system (BACTEC, MGIT, ESP); the latter allows more rapid culture and isolation of a greater range of species than does the use of solid media alone (3).
NTM are identified by their pattern of pigmentation, growth characteristics, microscopic appearance, and biochemical reactions. More rapid discriminating systems are being developed, and include DNA probes, high-performance liquid chromatography, polymerase chain reaction restriction enzyme analysis, and 16S ribosomal ribonucleic acid (rRNA) gene sequence analysis (2,3).
Susceptibility testing of NTM is more difficult and more controversial than that of M. tuberculosis. In general, the results of standard susceptibility tests are of little or no value in predicting clinical efficacy in NTM infections, and the provision of in vitro susceptibility results to clinicians is likely to be more confusing than helpful (2,3). Both the American Thoracic Society and the British Thoracic Society
have recommended that routine testing of the susceptibility of NTM to antituberculous drugs be discouraged (2,3).
have recommended that routine testing of the susceptibility of NTM to antituberculous drugs be discouraged (2,3).
Mycobacterium Avium Complex
MAC is the most commonly isolated and most clinically important pulmonary NTM pathogen, and includes the two species Mycobacterium avium and Mycobacterium intracellulare. The fact that they are distinct, however, has no clinical or prognostic value for individual patients. They are therefore considered together as MAC.
MAC pulmonary disease occurs in patients with chronic lung disease, with deficient cellular immunity, with AIDS, and also with increasing frequency in persons without apparent underlying disease. Early studies showed that patients with chronic lung disease or deficient cellular immunity accounted for >50% of cases of MAC pulmonary disease, whereas patients without predisposing factors accounted for approximately 25% of the total number of
HIV-negative cases (11). More recent studies have shown a steady increase in the incidence of MAC pulmonary disease in persons without underlying predisposing condition that currently accounts for >50% of cases of MAC pulmonary infection in HIV-negative cases (12).
HIV-negative cases (11). More recent studies have shown a steady increase in the incidence of MAC pulmonary disease in persons without underlying predisposing condition that currently accounts for >50% of cases of MAC pulmonary infection in HIV-negative cases (12).
The symptoms and signs of MAC lung disease are variable and nonspecific. Moreover, the natural history of MAC pulmonary disease in patients who are HIV-negative is unpredictable. Some patients show a stable clinical and radiographic picture for years, while others demonstrate a relatively rapid progression of the disease. This feature appears to relate in part to the existence of two main types of clinical disease and presentation.
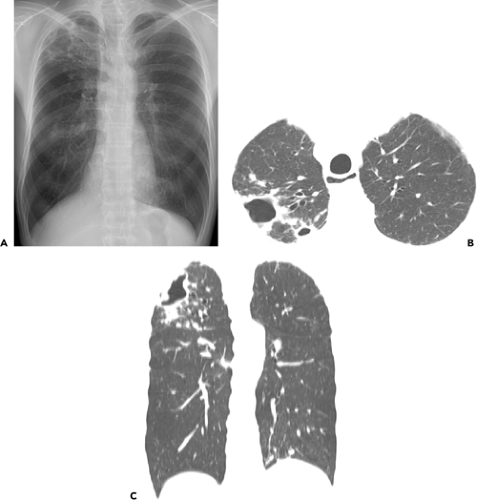
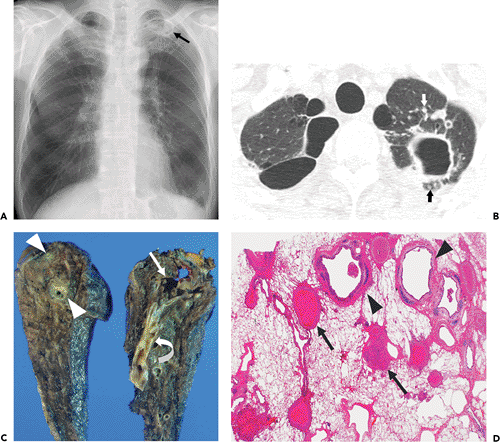
The two main patterns of MAC pulmonary disease are the upper lobe cavitary form (see Figs. 4.1 and 4.2) and the nodular bronchiectatic form (3) (see Fig. 4.3). The former is usually seen in white, middle-aged or elderly men who smoke or abuse alcohol. Common underlying pulmonary disorders include chronic obstructive pulmonary disease, previous tuberculosis, and silicosis. Chest radiography frequently demonstrates apical cavitary changes similar to those seen in postprimary
tuberculosis (13,14,15). Cavitation is common and frequently associated with apical pleural thickening. The cavities are usually thin-walled (16,17) (Figs. 4.1 and 4.2). Endobronchial spread of disease is common and manifests as unilateral or bilateral small ill-defined nodules on chest radiograph. High-resolution CT scan shows the nodules to be centrilobular and frequently associated with branching opacities (tree-in-bud pattern). Upper lobe fibrosis with volume loss and traction bronchiectasis occurs in one-third of patients (13,14,15). Lymphadenopathy and pleural effusion are uncommon. This form of disease is generally progressive, and if left untreated, can lead to extensive lung destruction and eventually, death (3).
tuberculosis (13,14,15). Cavitation is common and frequently associated with apical pleural thickening. The cavities are usually thin-walled (16,17) (Figs. 4.1 and 4.2). Endobronchial spread of disease is common and manifests as unilateral or bilateral small ill-defined nodules on chest radiograph. High-resolution CT scan shows the nodules to be centrilobular and frequently associated with branching opacities (tree-in-bud pattern). Upper lobe fibrosis with volume loss and traction bronchiectasis occurs in one-third of patients (13,14,15). Lymphadenopathy and pleural effusion are uncommon. This form of disease is generally progressive, and if left untreated, can lead to extensive lung destruction and eventually, death (3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree