CHAPTER 6 Michael P. Riley, MD, PhD, and Andrew E. Epstein, MD First described in 1998, catheter ablation for the treatment of atrial fibrillation (AF) has become widely performed worldwide.1,2 Over the years, the procedure has evolved in several ways, including refinement of patient selection, improved techniques used to perform the procedure, and better management of anticoagulation, all which have improved outcomes.3,4 This chapter will describe the most up-to-date information about each of these issues. Patients who undergo catheter ablation typically fall into one of two groups: (1) patients who experience symptoms due to AF, or (2) patients who belong to the minority of those who have left ventricular dysfunction due to tachycardia-induced cardiomyopathy from AF.4–6 Within these groups, appropriate selection for AF ablation should be on the basis of a combination of two factors: (1) the likelihood for a successful outcome in terms of durable restoration of sinus rhythm, and (2) the likelihood that the patient will tolerate the procedure and be free of complications. Emerging indications include combining AF ablation with cardiac resynchronization therapy to enhance biventricular pacing and to decrease the risk of inappropriate shocks.7 Regarding the likelihood for a long-term successful procedure, it is important to contrast two of the essential forms of AF: paroxysmal AF and persistent AF. Patients with the paroxysmal form of AF, whose rhythm spontaneously transitions between AF and sinus, typically have better outcomes than patients with persistent or permanent AF with ablation, and are, therefore, more likely to be selected for the procedure. This increased likelihood of success occurs because the ablation procedure is best at eliminating or modulating the triggering beats that initiate AF. In most cases, these triggers originate within the pulmonary veins, and the ability to electrically isolate the pulmonary veins from the rest of the atria is the reason for this success. Patients with persistent AF, who remain in AF for long periods of time unless cardioversion is performed, can also be successfully treated with ablation, but the success rate is lower, probably because of atrial structural changes that increase susceptibility to recurrences and result when AF is persistent.8,9 As such, despite myriad ablation strategies that have been employed over the years, there remains much to be learned about the structural differences between paroxysmal and persistent AF, and the best ablation method(s) to treat these two forms to improve ablation outcomes, especially for persistent AF. Indeed, patients with long-standing persistent or permanent AF who have remained in continuous AF for long periods of time are some of the more challenging patients to treat. Furthermore, patients with structural heart disease, such as hypertrophic cardiomyopathy, rheumatic heart disease, severe mitral insufficiency, or stenosis can be challenging to ablate in terms of durable success, again likely the result of structural remodeling in the left atrium. Additionally, the presence of obstructive sleep apnea also is a strong risk factor limiting long-term success of ablation.10 The literature provides guidance with regard to choosing patients likely to tolerate the procedure and be free of complications. Many older patients (>80 years of age) tolerate AF ablation well and have successful outcomes, but younger ones (<50 years of age) rarely experience complications.11–16 The complications associated with AF ablation are well known, and though infrequent, can be serious (Table 6.1).2,17,18 Patients with comorbidities including obesity, renal insufficiency, and advanced pulmonary disease can take longer to recover from the procedure and may be at increased risk in terms of their ability to tolerate a complication.
Nonpharmacologic Approaches to Rhythm Control—Ablation
PATIENT SELECTION
Table 6.1 |
1. Stroke 2. Hemorrhagic pericardial effusion necessitating percutaneous or surgical drainage 3. Phrenic nerve palsy and diaphragmatic dysfunction 4. Pulmonary vein stenosis 5. Atrioesophageal fistula 6. Major vascular complication, for example, retroperitoneal bleeding |
TECHNIQUES
The techniques used for AF ablation have evolved in several ways since the procedure was initially performed. Electrical isolation of the pulmonary veins remains the cornerstone of the procedure. Initially, this isolation was performed by delivery of radiofrequency ablation lesions at the ostia of the pulmonary veins.19 More recently, ablation is typically delivered more proximal to the pulmonary veins in a more antral region as the pulmonary veins transition to the left atrium (Figure 6.1). A number of catheters are available for ablation, and one study has shown that although tip design does not affect total lesion volume, there are differences in lesion geometry in terms of surface diameter and depth. Furthermore, the catheters may have different safety profiles.20 Newer ablation tools designed to achieve pulmonary vein isolation have included balloon technologies designed to fit into the pulmonary vein ostia and occlude blood flow from the pulmonary vein.21–23 In this way, a tight seal is achieved between the balloon and the pulmonary vein ostia, allowing for the delivery of one of several potential forms of energy to electrically isolate the pulmonary vein. Table 6.2 lists some of the forms of energy that have been used to achieve pulmonary vein isolation by using balloon technology. There is some concern that some of the newer ablation technologies may have a small increase in the rate of periprocedural complications owing to the way that they deliver energy to achieve pulmonary vein isolation. Methods to decrease the risk of one of the most feared complications, production of an atrio-esophageal fistula, include modifying energy output, visualizing the esophagus by MRI, echocardiographic, or swallowed contrast, and monitoring esophageal temperature.24,25
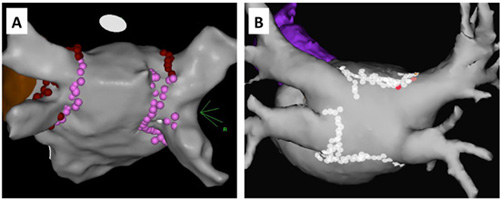
Examples of 2 different left atrial lesion sets around the pulmonary veins. A: A posterior view of the left atrium with a lesion set about the left pulmonary veins and a separate lesion set around the right pulmonary veins. B: A posterior view of the left atrium but with a wider set of lesions that surround the entire pulmonary veins in one lesion set.
Table 6.2 |
1. Radiofrequency energy delivered in focal lesions 2. Cryoablation energy delivered via an occlusion balloon or via focal lesions 3. Focused ultrasound delivered via an occlusion balloon 4. Radiofrequency energy delivered along a circular ablation catheter 5. Laser energy delivered via an occlusion balloon |
In addition to pulmonary vein isolation, some electrophysiologists have investigated whether additional ablation lesions delivered away from the pulmonary veins could improve the outcomes associated with the procedure.26–29 Table 6.3 lists some of the various additional lesions sets that have been suggested. There remains no clear consensus on whether additional ablation is worthwhile and if so, which lesions sets provide the greatest efficacy. In addition, the drawbacks of potentially unnecessary additional ablation include increased risk of complications, radiation exposure, length of the procedure, and potential proarrhythmic effects in terms of atrial flutters that can be promoted by the creation of regions of slow conduction in areas of incomplete ablation. There are relatively few end points for an AF ablation but they include the following: pulmonary vein isolation, termination of AF, and noninducibility of AF. Pulmonary vein isolation is best assessed by the absence of electrical conduction into or out from the pulmonary vein, also referred to as entrance and exit block, respectively. Durable conduction block should prevent any pulmonary vein triggers from reinitiating AF. The end point of termination of AF is not pursued by every operator performing AF ablation, and is typically sought by those performing more extensive ablation beyond pulmonary vein isolation.30 The hypothesis underlying this end point is that ablation that results in AF termination may have led to ablation at sites beyond the pulmonary veins that are critical for the maintenance of AF in those patients with more persistent forms of AF. Noninducibility of AF may be the optimal end point for ablation. If, despite aggressive stimulation with either rapid pacing or catecholamine boluses, AF cannot be reinduced, this may portend a good outcome.31
Table 6.3 |
1. Pulmonary vein isolation 2. Lines of ablation lesions delivered between two electrical barriers a. “Roof” line connecting left and right superior pulmonary veins b. “Mitral isthmus” line connecting left inferior pulmonary vein and mitral annulus c. “Superior mitral isthmus” line connecting right superior pulmonary vein and mitral annulus d. “Cavo-tricuspid isthmus” line connecting inferior tricuspid annulus with inferior vena cava 3. Ablation at sites of complex fractionated electrograms 4. Ablation at sites of common non-PV triggers 5. Ablation at sites of rotory electrical activity 6. Ablation in either the right or left atria intended to decrease left atrial muscle mass |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


