Fig. 7.1
CT angiographic images in a 78-year-old woman with chronic mesenteric ischemia. Sagittal oblique maximum intensity projection image (a) demonstrates occlusion of the celiac (solid arrow) and superior mesenteric (dashed arrow) artery origins. Volume-rendered image (b) demonstrates a markedly enlarged marginal artery of Drummond (arrowheads) from the inferior mesenteric artery supplying the celiac and superior mesenteric circulation
Although CTA is generally considered first-line imaging in cases of suspected chronic mesenteric ischemia, MRA is useful in equivocal cases or patients who have contraindications to CT contrast. MRA utilizing either specific gadolinium contrast agents or noncontrast techniques can also be advantageous to avoid potential complications of allergic reaction or contrast-induced nephropathy. MRA with contrast is well suited for the visualization of the proximal mesenteric arteries with an accuracy that is comparable to CTA [13]. Some authors have suggested that given the higher spatial and temporal resolution of CTA versus MRA, the IMA and distal mesenteric vessels are better assessed on CTA [13]. In our experience, with state-of-the-art MR techniques, non-visualization of a patent IMA or major SMA branches for technical reasons is very rare (Fig. 7.2), but with older scanners and less experienced operators, this certainly could still be a problem in routine clinical practice. Limited evaluation of the distal mesenteric vasculature is also less of a hindrance in the evaluation of chronic than acute mesenteric vascular disease.
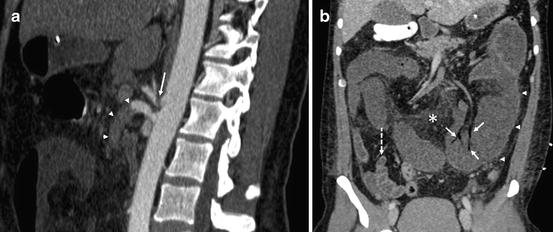

Fig. 7.2
A sagittal oblique MPR image (a) in a 40-year-old male with median arcuate ligament compression of the celiac axis (arrow) and an embolus lodged in the superior mesenteric artery (arrowheads). Coronal delayed phase CT image (b) demonstrates evidence of bowel ischemia with dilated, poorly enhancing loops of jejunum in the left mid-abdomen (arrowheads, contrasted with normal ileum in the right lower quadrant with a dashed arrow) and gas in portal venous tributaries (arrows). Also evident is mesenteric venous fat stranding indicating inflammation (asterisk)
In addition, MRA can be combined with functional physiologic data, which may additionally help to support or refute the diagnosis. Utilizing normal healthy volunteers, taking pre- and postprandial flow measurements, MR flow quantitation has demonstrated a postprandial increase in blood flow in the postprandial SMA and SMV due to the increased metabolic demands of the gut [14]. Studies have shown a correlation between the degree of SMA stenosis and the blunting of the normal postprandial blood flow increase in patients with varying degrees of SMA stenosis [16]. Other studies have shown a markedly decreased postprandial response in SMV flow in patients with chronic mesenteric ischemia versus healthy individuals [18]. Most commonly, flow in the SMV is measured prior to feeding and then at 15, 30, 45, and 60 min following a meal challenge. Increased flow in the SMV less than 50 % above baseline is considered abnormal and supportive of the diagnosis of chronic mesenteric ischemia [14, 16].
MR oximetry measures differences in T2 relaxation time of oxy- and deoxyhemoglobin. In normal healthy individuals, blood flow to the gut is increased following a meal to meet increased metabolic demands. In patients with stenosis or occlusions of the mesenteric arteries, blood flow cannot be increased, and therefore, oxygen extraction must be increased to attempt to meet the metabolic demand of the gut. Similar to flow quantitation, oximetry is performed in the SMV in the preprandial state and following a meal challenge. Studies have shown that in normal individuals the oxygen saturation in the SMV increases by about 5 % following feedings. In symptomatic patients with angiographically proven stenosis of two of the three mesenteric arteries, postprandial oxygen saturation decreases by about 9 % [14, 19].
Acute Mesenteric Ischemia
The major causes of acute mesenteric ischemia include embolus, mesenteric arterial thrombosis, mesenteric venous thrombosis, and nonocclusive mesenteric ischemia [8, 13, 14]. Acute embolus to the SMA is the most common cause of acute mesenteric ischemia, occurring in 40–50 % of the cases [13]. Most emboli to the SMA tend to lodge a few centimeters from the origin, just distal to the middle colic artery ostium. Smaller emboli can lodge further distally in the smaller splanchnic branches [13]. Nonocclusive emboli are visible on CTA as low-density filling defects within the vessel lumen, and occlusive emboli are visible as an abrupt termination of the vessel (Fig. 7.3). Given the acute nature of embolic phenomena, collateral vessels are frequently absent. Acute mesenteric artery thrombosis is the second most common cause of acute mesenteric ischemia, accounting for 20–30 % of all cases [13]. Thrombosis occurs following the rupture of an unstable atherosclerotic plaque leading to the accumulation of platelets and inflammatory mediators. Given that the thrombosis usually occurs in the setting of preexisting atherosclerotic disease, the site of thrombosis is typically at the origin of the SMA or within the proximal 2 cm [8]. Many patients presenting with acute SMA thrombosis have long-standing atherosclerotic disease, and their bowel is subjected to chronic ischemia. This leads to the development of small arterial collateral vessels, which can be readily seen on CTA. The typical imaging findings in SMA thrombosis are the complete occlusion of the origin or proximal SMA with poor visualization of the distal branches. This is in distinction to emboli, which tend to affect more distal branches. Intraluminal filling defects are uncommon in acute thrombosis [8]. Nonocclusive mesenteric ischemia and venous thrombosis together account for approximately 30–40 % of cases of acute mesenteric [8].
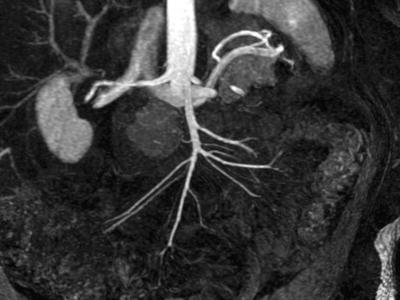

Fig. 7.3
Thin maximum intensity projection from a contrast-enhanced MR angiogram in a 55-year-old woman with a prior superior mesenteric artery (SMA) dissection demonstrates clear visualization of normal-appearing SMA and multiple jejunal and ileal branches
No matter the cause of the mesenteric ischemia, the sequela of bowel ischemia can be readily assessed with CTA. Hypoperfused bowel exhibits decreased peristalsis and retention of fluid and secretions. This leads to small bowel dilation (>3 cm), colonic dilatation (>9 cm), or bowel obstruction, with geographically dilated bowel. Edema and poor perfusion within the bowel wall commonly cause low attenuation and circumferential thickening up to 1.5 cm [8]. This severe degree of bowel thickening is more commonly seen in venous thrombosis than in arterial inflow causes, which tend to have bowel wall thickening of 8–9 mm [8] (Fig. 7.4). Occasionally, the bowel wall can be normal or even thinned. Although decreased contrast inflow and mural edema can give the appearance of low density and hypoenhancement; hemorrhage within the bowel wall or compensatory hyperemia may paradoxically cause bowel wall hyperattenuation. Pneumatosis intestinalis is seen late in the course of ischemia, indicating transmural infarction with mucosal breakdown. This finding is commonly accompanied by gas within the portomesenteric system and carries a poor prognosis.
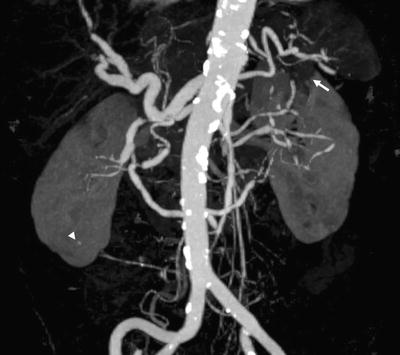

Fig. 7.4
Coronal oblique maximum intensity projection image from contrast-enhanced CT angiography in a 79-year-old female patient with a two-week history of abdominal pain, nausea, and anorexia. The exam was performed for suspicion of mesenteric ischemia. The image demonstrates diffuse segmental ectasia and stenotic “beading” of the mesenteric and renal circulation, as well as a small segmental infarct in the upper pole left kidney (arrow) and a tiny intrarenal aneurysm on the right (arrowhead). The findings are compatible with polyarteritis nodosa, and the patient’s symptoms resolved on steroid therapy
Cross-sectional imaging allows the evaluation of organs other than the bowel within the abdomen and pelvis, which aids in the differential diagnosis of conditions that can mimic acute mesenteric ischemia. Entities frequently seen on CTA in the absence of mesenteric ischemia include small bowel obstruction, cholecystitis, and diverticulitis [20, 21].
In the absence of definitive vascular findings, other findings can be seen due to decreased blood flow within the bowel. Mesenteric fat stranding or congestion, ascites or free abdominal fluid, and free abdominal air are generalized signs of a number of abdominal processes that can also be seen in mesenteric ischemia. Unfortunately, many of these secondary findings can be seen in a wide range of nonischemic conditions and lack specificity. The most common findings seen on CT in patients with acute mesenteric ischemia are mesenteric fat stranding, bowel wall thickening, bowel dilatation, and ascites [20–22]. These findings are suggestive of acute mesenteric ischemia, although their specificity ranges from 20 to 86 %. The most specific findings were those of late complications, including pneumatosis intestinalis, solid organ infarctions, and portal venous gas with specificities of 100 % reported in multiple studies [21, 22]. In an attempt to optimize the algorithm for diagnosis of acute mesenteric ischemia utilizing MDCT, one group diagnosed mesenteric ischemia with the single finding of pneumatosis intestinalis, portomesenteric venous gas, or visceral artery occlusion, or a combination of bowel wall thickening with portomesenteric venous thrombosis or solid organ infarction. This approach yielded a sensitivity of 93 %, specificity of 100 %, positive predictive value of 100 %, and negative predictive value of 96 % [21].
The use of MRA performed with intravenous contrast has a limited role in the diagnosis of acute mesenteric ischemia. MRA has a high accuracy in diagnosing arterial embolism or thrombosis of the proximal vasculature, but is usually more limited for evaluation of distal branches. Given the long acquisition times required for multiple sequences and the lack of widespread availability, the use of MRA may delay intervention in an acutely ill patient [14, 23]. In select cases, such as those patients with a severe iodine allergies or renal insufficiency, contrast-enhanced or noncontrast MRA may be a reasonable option.
Mesenteric Venous Thrombosis
Mesenteric venous thrombosis accounts for 5–15 % of cases of mesenteric ischemia [13]. The presentation can be variable and can occur as either an acute or a chronic disorder with patients clinically ranging from asymptomatic to critically ill, depending on the acuity and occlusiveness of the thrombosis [24]. The thrombus is seen as a filling defect at the site of venous obstruction, which can propagate peripherally. In cases attributable to a hypercoagulable state, venous thrombus is often first seen in the smaller branches as a low-density intraluminal filling defect and then spreads centrally towards the major venous trunks [24]. 3D reconstructions are especially useful for imaging the mesenteric venous system as these vessels can be easily identified and followed on the coronal and coronal oblique projections [10]. The confluence of the superior mesenteric vein and portal vein is a common site for venous thrombosis, and coronal reconstructions permit quick and accurate assessment of this area [8]. Several authors have found that other reconstructions, such as maximum intensity projections using thin slabs, are best used to display venous collaterals that occur in the presence of mesenteric venous thrombosis [8]. Due to the venous collateral system of the bowel, patients can be asymptomatic, even with a large degree of venous thrombosis, and evidence of bowel ischemia on CT or MR may not be present in isolated thrombosis. In acute or complete thrombosis, without robust venous collateral flow, severe venous hypertension results lead to engorgement of the veins with wall thickening and enhancement [13]. The bowel wall can be significantly thickened and congested due to venous obstruction. Intramural hemorrhage can be seen within the bowel wall as increased density on CT. With long-standing venous obstruction, transmural infarction can occur, which can cause complete lack of bowel wall enhancement [8]. This can lead to findings of pneumatosis intestinalis or portomesenteric gas, which portend a poor prognosis [8, 13] (Fig. 7.4). The generalized abdominal findings of bowel dilatation or ascites fluid are nonspecific and can be seen in venous thrombosis; however, hemorrhagic peritoneal fluid appearing hyperdense on CT suggests early bowel infarction [25].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


