Fig. 17.1
Application of the vacuum bell

Fig. 17.2
Vacuum bell in three different sizes (left 16 cm, middle 19 cm, right 26 cm in diameter)
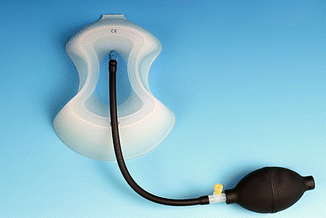
Fig. 17.3
Vacuum bell, model for adolescent girls/women
Indication and Contraindication
Indication for conservative therapy with the vacuum bell include patients who present with mild degree of PE and/or who want to avoid surgical procedure. In particular patients under the age of 10 years with a still flexible and elastic chest wall represent good candidates to start with the application.
Contraindications of the method comprise skeletal disorders, vasculopathies, coagulopathies and cardiac disorders [38]. To exclude these disorders, a standardised evaluation protocol was routinely performed before beginning the therapy.
Complications and relevant side effects include subcutaneous hematoma, petechial bleeding, dorsalgia and transient paresthesia of the upper extremities during the application.
Methodology
All patients who visit our specialized outpatient clinic for chest wall deformities are informed about the option of conservative vs. surgical therapy to correct PE. Standardised evaluation includes history of the patient and his family, clinical examination and photo documentation of the PE. The depth of PE is measured in a standardized supine position. When the patient and/or the parents decide to perform the conservative vacuum therapy, meticulous family history is necessary to capture the above-mentioned contraindications. To exclude cardiac anomalies, we do routinely cardiac evaluation with electrocardiogram and echocardiography before starting the daily application in every patient.
Conservative Treatment
The first application of the vacuum bell occurs during the outpatient clinic visit under supervision of the attending physician. The appropriate size and model of the different type is defined. Patients learn the proper application of the device. In children under the age of 10 years, parents are instructed to use the device and children apply the vacuum bell under supervision of their parents or caregivers, respectively. The middle of the window should be positioned above the deepest point of the PE. Starting the application, the hand pump should be activated with 2–3 pumps. Patients are usually in a supine position for the first application. During therapy, most adolescent and adult patients apply the device in an upright position whereas parents of children under the age of 10 years prefer to continue in a supine position. With the device in position, patients may move and walk around in their home environment.
In patients with a localized deformity, it could be helpful to apply the device using the small model. In patients with an asymmetric PE or a grand canyon type PE, it could be useful to apply the device in changing positions.
When cardiac anomalies and other contraindications are excluded, patients may start with the daily application. All users are recommended to start to use the device twice daily for 30 min each. During follow-up, some patients follow the user instructions applying the device twice daily for 30 min each. However, some of the adult patients use the vacuum bell up to 8 h daily during office hours. A group of adolescent boys apply the device every night for 7–8 h. Since there does not yet exist a detailed study protocol for the application, the duration and frequency of daily application depends on the patients’ individual decision and motivation.
Patients undergo follow-up at 3–6 monthly intervals including clinical examination, measurement of depth of PE and photo documentation. Clinical examination focuses on the improvement of depth of PE as well as on relevant side effects such as persistent hematoma and/or skin irritation. If necessary, tips and tricks to optimize the application are discussed. The endpoint of therapy is defined by the patient’s individual decision, which is confirmed by our clinical examination during the routine outpatient clinic visit. In addition to the daily vacuum bell application, all our patients are recommended to carry on undertaking sports and physiotherapy, so that the accompanying improvement of body control results an important factor in outcome.
Patients
Our patients group comprises applicants aged from 3 to 61 years. As mentioned previously, we observed age specific differences of success [39], and therefore the most favourable age for this treatment has still to be defined.
During the first few applications, most of the patients experience moderate pain in the sternum. Adolescent and older patients develop moderate subcutaneous hematoma, which disappears within a few hours. Temporary side effects like transient paresthesia of the upper extremities during the application and/or mild dorsalgia are reported by some patients. These symptoms disappear when lower atmospheric pressure is used during application. Analgesic medication should not be necessary and has not been reported from any patient and parents, respectively. As mentioned above, the application of the vacuum bell in children aged 3–10 years should be supervised by parents or caregivers.
Results
Within the last 11 years, 300 patients (62 female, 238 male) started with vacuum bell treatment at our institution. The median age was 16.2 years (3–61 years). When starting with the application, 67/300 patients were above the age of 17 years, 58/300 above the age of 18 years. We published preliminary results of a subset of our patients group in 2011 [38]. Latest and more detailed results were summarized in another published study [39]. Hundred and forty patients (112 males, 28 females), aged 3–61 years (median 16.05 years) used the vacuum bell for 6 to maximum 69 months (average 20.5 months). When starting with the application, patients presented with a PE with depth from 1 to 6.3 cm (average 2.7 cm). After 3 months of treatment an elevation of more than 1 cm was documented in approx. 80 % of patients.
Daily application of the whole group was 107.9 min/day (range, 10–480 min). Application was terminated after 20.5 months. In 61 patients, the sternum was lifted to a normal level after 21.8 months (range, 6–69 months) (Figs. 17.4 and 17.5).
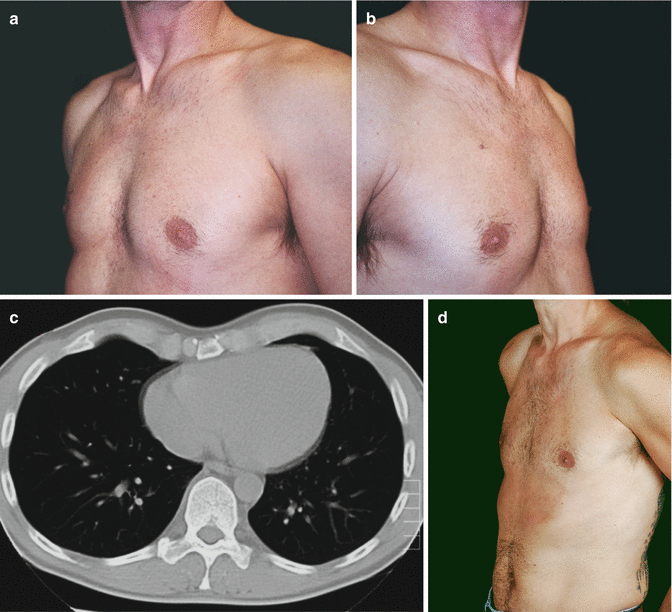
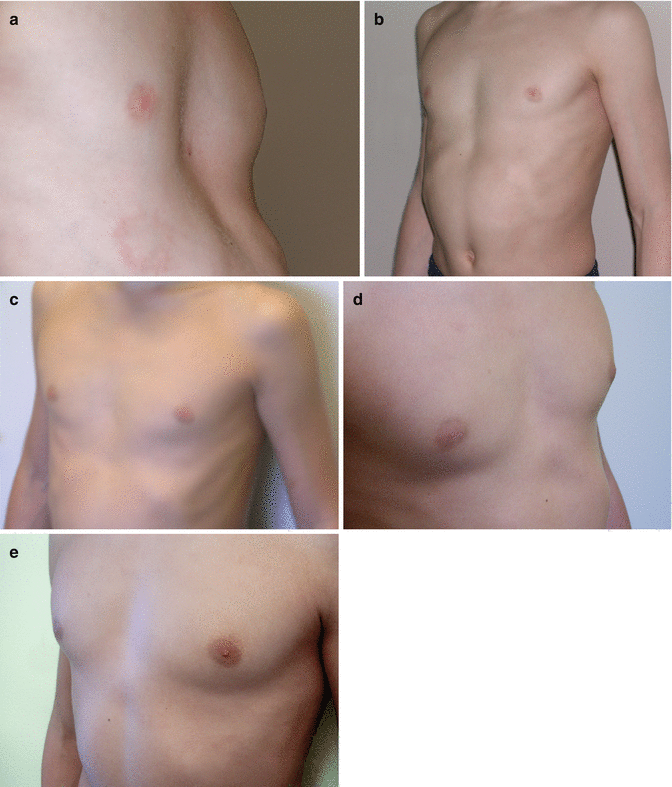


Fig. 17.4
Forty-five-year-old patient. (a–c) before (left: depth of PE = 2.5 cm) vacuum bell therapy and (d–f) after 12 months (right: depth of PE = 0.5 cm)

Fig. 17.5
Nine year old boy, (a) before (left: depth of PE = 2.8 cm) vacuum bell therapy, (b) after 10 months (right: depth of PE = 1.6 cm), (c) after 16 months (depth of PE: 0.4 cm), (d) 24 months after therapy and (e) 36 months after therapy
The follow-up after discontinuation is 27.6 months (range, 1–73 months), and the success until today is permanent and still visible (Figs. 17.4 and 17.5). Patients were very well motivated and compliant which is a basic precondition for a successful therapy. At follow-up, all patients were satisfied and expressed their motivation to continue the application, if necessary. Fifty-four patients are still under treatment. However, 25/140 patients stopped the application after 15.7 months (range, 1–42 months), due to an unsatisfactory result and/or decreasing motivation. 15/25 patients underwent MIRPE. The relevance of motivation was confirmed by the fact that 15 patients who underwent MIRPE, used the vacuum bell for 160.6 min/day whereas the remaining 10 patients who stopped any kind of therapy, used the vacuum bell for 36.3 min/day. In three patients with asymmetric PE, the depth of PE has decreased after 9 months, but the asymmetry is still visible (Fig. 17.6).
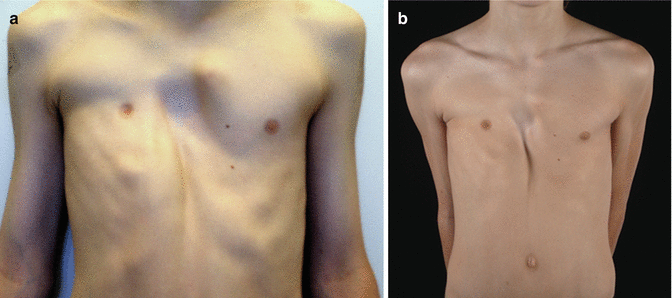

Fig. 17.6
Ten year old boy with asymmetric PE, before (a) vacuum bell therapy, and after 12 months (b)
Intraoperative Use
Our experience with the vacuum bell method encouraged us to use the device intraoperatively during the MIRPE procedure to facilitate the dissection of the transmediastinal tunnel and the advancement of the pectus introducer, the riskiest step of the MIRPE procedure. As already demonstrated by Schier and Bahr for the first time [20], the elevation of the sternum is obvious and persists for a distinct period of time after application of the vacuum bell. Therefore, we considered that the vacuum cup may also be useful in reducing the risk of injury to the heart and the mammary vessels during the MIRPE procedure. Since the manufacturer of the device did not apply for the approval to sterilize the vacuum bell until today, this additional use had to be considered as “Off-label”. In agreement with our hospital hygienist and bearing in mind the nature of the material, we used gas sterilization for preparation of the device for intraoperative use.
Results
In a pilot study performed from 2005 to 2010, 50 patients aged from 9 to 28 years (average 14.95 years; 39 males and 11 females) were operated on for PE using the MIRPE procedure. Thirty-eight patients underwent primary surgery. Twelve patients (11 male, 1 female) used the vacuum bell for a period of 4–36 months (average 19.9 months) before surgery, and discontinued the application due to decreasing motivation and/or insufficient success. The vacuum bell was applied for retrosternal dissection and advancement of the pectus introducer as well as placement and flipping of the pectus bar. The use of the vacuum bell led to a clear elevation of the sternum and this was confirmed by thoracoscopy (Fig. 17.7). Advancement of the pectus introducer and placement of the pectus bar was safe, successful and without adverse events in all patients. No evidence of cardiac and/or pericardiac lesions or lesions of the mammary vessels were noted intraoperatively by using right sided thoracoscopy. Additionally, no midline incision to elevate the sternum with a hook was necessary [22].
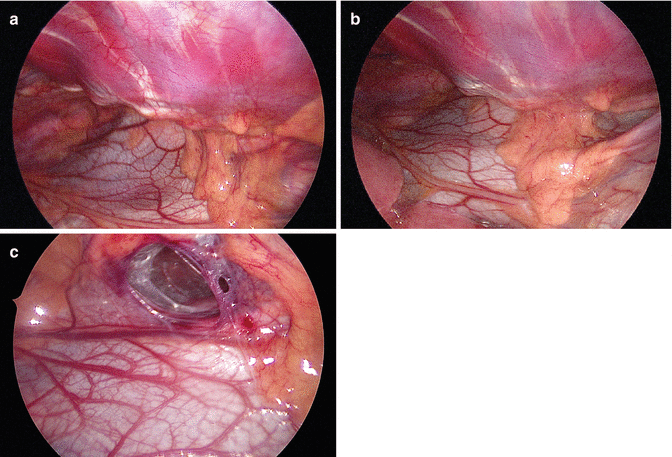

Fig. 17.7
Retrosternal space creation without (a) and with the vacuum bell (b) during the MIRPE procedure. (c) Note the retrosternal tunnel (R)
Discussion
A more differentiated analysis of our patients group will enable us “to see behind the curtain”. Age and gender specific differences, depth of PE, symmetry or asymmetry, concomitant malformations like scoliosis and/or kyphosis, etc. may influence the clinical course and the success of this therapy. The influence of individual motivation on the success has been described above.
However, there still remain some unanswered questions:
Optimal age for vacuum bell therapy
The optimal age for this treatment has still to be defined. We observe age specific differences of success. In our experience, growth spurt during puberty is the most important period to influence degree and depth of PE. We started a pilot study using a measuring device which might enable us to measure the correlation between patients age, the depth of PE and the elevation of the chest wall during application. With these results, we may evaluate whether beginning with the vacuum therapy before puberty will be more useful than starting during puberty or even later.
Quantitative Measurement of Pressure
The success of a therapeutic procedure not only requires a good technique, but also depends on an appropriate indication. It would be useful to measure the pressure that is necessary to lift the sternum during the first application. This measurement would enable us to divide patients into different groups, to identify suitable patients, and allow us to predict more accurately who of the users will benefit from this method and in whom the method will not work. As mentioned above, we are working on such a device to measure the pressure under the vacuum bell.
Supervision of daily application
Until today, we have no possibility to supervise the frequency, the intensity and the duration of the daily application at home. Electronic devices which might be integrated into the vacuum bell, would be useful to supervise the routine application.
Long-term Results
Long-term results including 10 years and more are still missing. Further studies are necessary to elucidate these facts.
Costs of Treatment
In most European countries, costs of treatment have to be paid by patients and parents, respectively. In some countries in South America, acquisition of the vacuum bell is covered by the individual national health care system or the local insurance. In USA, approval of the FDA was obtained in May 2012.
Pre-Treatment before Surgery
Physicians and patients discuss about the benefit to use the vacuum bell preoperatively prior to MIRPE procedure. Since in our country the majority of patients have to pay for the device, most of our patients are not interested in this “pre-treatment”. Additionally, we observed no significant difference between patients who used the vacuum bell before surgery, and patients who underwent primary surgery [22].
Objective Assessment of Depth of PE
To estimate the “objective” success of this treatment modality is very difficult. The definition of success may vary considerably between individuals. Depth of PE, symmetric vs. asymmetric deformity, as well as patients’ age and sex represent important variables. Various scales and measurement methods including X-rays and computed tomography have been used to quantify the degree of deformity. Our method of assessment of depth of PE is not exact enough, especially regarding the age specific differences. New methods for non-invasive assessment of chest wall growth may provide more detailed, objective information concerning the severity of PE. A 3-D laser scanner might help us to assess the degree of PE and to follow-up our patients during vacuum therapy.
Pectus Carinatum
Introduction
PC is more frequent in males than females (4:1 ratio) and can be both symmetric or asymmetric. Rarely, the defect might be associated to Currarino-Silverman, combined pectus carinatum/excavatum, and Poland, Marfan or Von Recklinghausen syndromes, among other connective tissue disorders. Even though its etiology is unknown, PC may be genetically linked considering its frequent occurrence in families [40].
Apart from the external appearance which most commonly concerns patients and families, the majority of children present with relatively mild symptoms; the most frequently reported are tenderness, bone pain or mild exercise intolerance. Even though psychosocial issues secondary to body image need to be promptly addressed in all cases, since the defect tends to become more severe during pubertal growth spurts, and may even worsen throughout adult life, the physiological concerns must take precedence without exception.
Despite the early work of Jaubert de Beaujeu et al. and Bianchi et al. [41, 42], the pioneers in non-operative treatments for PC – open surgery has been the treatment of choice over the last decades [2, 43, 44]. Most of the existing surgical procedures consist of modifications of the Ravitch technique that employ resection of the deformed costal cartilages along with sternal osteotomy [45]. Even though patients refer to be generally pleased with the improvement of their chest’s shape, surgery could not address the usual problem of the flaring of ribs and a visible scar was always left. On top of that, it is well known now, that surgery does not result in complete thorax remodeling in comparison to non-operative treatments. Many different authors proposed less radical resections [46–48].
Drs. Haje DP, Haje SA and coworkers from Brazil, have shared their valuable, extensive experience in treating PC patients using a Dynamic Compressor System (DCS) [49–52]. This was the consequence of four basic facts: (1) the inherent risks of a major surgery, (2) always reserved for the most severe cases, (3) leaving a great deal of patients untreated [53], in addition to (4) anterior chest wall compliance during puberty which permits remodeling by applying external compression. Based on the latter, other authors have also suggested a wide variety of alternative non-operative approaches [10–16], too.
The FMF® Dynamic Compressor System
Foreword
The Nuss procedure for pectus excavatum introduced a paradigm shift by demonstrating that the thoracic wall is a very elastic and malleable structure in children [9]. Inspired by this concept, early in the year 1999, the author and his partner, began assessing chest wall compliance in patients with mild to moderate forms of PC by applying manual compression to the defect. Since it could be corrected without pain, a non-operative prospective study was designed and implemented (after being approved by the institution’s Research Ethics Committee) at the chest wall deformities outpatient clinic. A DCS was developed and utilized for this purpose. Besides, since by that time, there were no reports about the record and analysis of pressure measurements to compare series of patients, further investigation was done on that particular topic. By the beginning of 2001, the DCS design was finished. In 2008, the initial experience with the so-called FMF® Dynamic Compressor System (FMF stands for Fraire/Martinez-Ferro) was published [17–19]. Two quantifiable variables were defined to statistically compare objective data, collected at every consultation:
Pressure of Correction (POC): the pressure applied to the patient until the proper shape of the thorax is achieved. Basically, it is an indirect parameter to measure and quantify the patient’s chest wall flexibility. It is reduced throughout the treatment and is measured initially (because one of the inclusion criterions for bracing is that the POC ought to be equal or less than 14 PSI to prevent treatment failure) and at every consultation (Fig. 17.8).
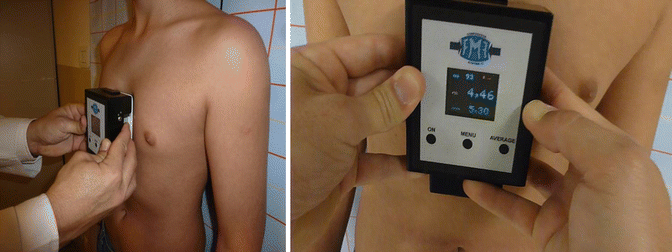
Fig. 17.8
Measurement of the Pressure Of Correction (POC) at the first and successive consultations. The patient stands up against the wall. The pressure measuring device (PMD) is placed over the chest, where the protrusion is more prominent, and measures the pressure required to remodel the thorax. The initial POC helps to predict treatment duration and indirectly quantifies thoracic flexibility
Pressure of Treatment (POT): the pressure required to treat the patient. It is measured before and after adjusting the FMF® DCS. POT permits evaluating whether the patient has been wearing the device or if he has grown up in between consultations. A POT higher than that obtained at the last consultation, means that he has not been wearing the brace as indicated, or that he has grown up (this can be verified by checking the registered height and weight or if the brace is too tight). Variables are recorded at an evolution form (Fig. 17.9).

Fig. 17.9
Measurement of the Pressure Of Treatment (POT). Before and after adjustments to the FMF® DCS. Note the three buttons for optimum performance and the digital multiparameter color display of the Pressure Measuring Device (PMD)
First Projects
At first, different kinds of plastic and then metallic orthotic devices that proved to be inefficient were developed. It could be noticed that when the patient’s thorax was compressed, it expanded laterally. The team concluded the reason for their failure was the fact that thoracic lateral expansion, occurring naturally during inspiration, had not been taken into account.
With the aid of mechanical and electronic engineers, an external DCS was designed, but this time loaded with an electronic Pressure Measuring Device (PMD) to measure the POC and POT. The PMD converts the mechanical energy exerted to the patient (pressure) into electrical energy visible as numbers in a screen (measurement of pressure).
The unit of measurement to quantify pressure was decided to be pounds per square inch (PSI) because it takes into consideration the pressure resulting from a force of one pound-force applied to an area of one square inch. Presently, this is the unit of pressure that is still being employed. Most of the patients can be included with a decimal scale from 1 to 14.
By that time, the FMF® DCS included an expandable aluminum brace and the PMD.
The initial results obtained from measurements of POC, age, time of usage and cosmesis were analyzed from prospective collected data. Surprisingly, by correlating the different variables, the authors found out they could predict treatment duration and prognosis. These data has been very useful since then, to assess the patient and family about the treatment from the very first day of consultation.
Throughout the following years, several modifications were introduced to the FMF® DCS. The posterior compression pad was removed as it was not useful and caused skin lesions upon the spine and dorsal tissue. Better tolerance from PC patients could be enhanced to complete the treatment. A docking mechanism was designed to attach the PMD to the brace (for regulation of POT), in addition to a locking system, to avoid patient manipulation, and a portable plate bender to model the aluminum pieces.
Today
The FMF® DCS is currently a system comprised of the following elements:
1.
Get Clinical Tree app for offline access

A custom-fitted, expandable, low-profile (invisible under the patients’ shirt), cushioned aluminum brace that is adjustable to any thoracic shape or size (Fig. 17.10). Its locking mechanism is situated on the side where the prominence is most evident to enhance compression. In order to avoid referrals, the brace has been designed to be ordered, assembled and implemented at different, distant locations with ease (Fig. 17.11). It permits lateral expansion to allow thorax widening as a consequence of breathing, growth and thoracic re-shaping (Fig. 17.12).


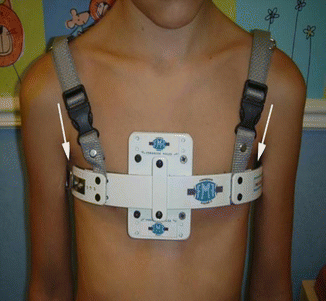

Fig. 17.10
Components of the FMF® Dynamic Compressor System: (left) Pressure Measuring Device (PMD) and (right) aluminum, lightweight brace

Fig. 17.11
Components of the FMF® Dynamic Compressor System: pieces to assemble a customized brace for each patient. Format in which they are delivered in a personalized packaging

Fig. 17.12




The FMF® DCS enables lateral expansion during breathing and thoracic widening that occurs with re-shaping and growth. Note that at least a 1 cm space is left on each thoracic side. Basically the skin should not be in direct contact with the brace’s undersurface to permit thoracic widening
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


