Chapter 12 Non-respiratory functions of the lung
 The entire cardiac output passes through the pulmonary circulation, so the lungs act as a filter, preventing emboli from passing to the left side of the circulation.
The entire cardiac output passes through the pulmonary circulation, so the lungs act as a filter, preventing emboli from passing to the left side of the circulation. The lungs constitute a huge interface between the outside environment and the body, requiring the presence of multiple systems for defence against inhaled biological and chemical hazards.
The lungs constitute a huge interface between the outside environment and the body, requiring the presence of multiple systems for defence against inhaled biological and chemical hazards.Filtration
Pulmonary capillaries have a diameter of about 7 μm, but this does not appear to be the effective pore size of the pulmonary circulation when considered as a filter. There is no clear agreement on the maximal diameter of particles that can traverse the pulmonary circulation. Animal studies have demonstrated the passage through perfused lungs of glass beads up to 500 μm.1 It is well known that small quantities of gas and fat emboli may gain access to the systemic circulation in patients without intracardiac shunting. Emboli may bypass the alveoli via some of the pre-capillary anastomoses that are known to exist in the pulmonary circulation (page 99), though the functional role of these anastomoses remains uncertain. More extensive invasion of the systemic arteries may occur in the presence of an overt right-to-left intracardiac shunt, which is now known to be quite common. Post mortem studies show that over 25% of the population have a ‘probe-patent’ foramen ovale, usually in the form of a slit-like defect that acts as a valve, and which is therefore normally kept closed by the left atrial pressure being slightly greater than the right.2 In 10% of normal subjects, a simple Valsalva manoeuvre or cough results in easily demonstrable blood flow between the right and left atria.3 Paradoxical embolism may therefore result from a relative increase in right atrial pressure caused by physiological events or pulmonary embolus (Chapter 29).
So far as the survival of the lung is concerned, the geometry of the pulmonary microcirculation is particularly well adapted to maintaining alveolar perfusion in the face of quite large degrees of embolisation. However, a significant degree of embolisation inevitably blocks the circulation to parts of the lung, disturbing the balance between ventilation and perfusion. This situation is considered in Chapter 29. Pulmonary microembolism with small clumps of fibrin and/or platelets will not have a direct effect on gas exchange until it is very extensive. Plugging of pulmonary capillaries by microemboli does, however, initiate neutrophil activation in the area, leading to an increase in endothelial permeability and alveolar oedema, and has been implicated in the aetiology of acute lung injury (Chapter 31).
Defence against Inhaled Substances
Airway Lining Fluid
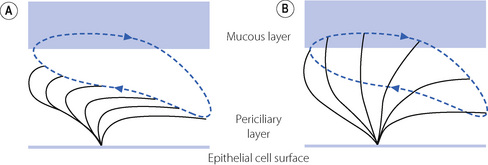
Within the airway lining fluid there are two distinct layers,4,5 a periciliary or ‘sol’ layer which is of low viscosity containing water and solutes and in which the cilia are embedded, and a mucous or ‘gel’ layer above.
Mucous layer. Large airways are completely lined by a mucous layer, whilst in smaller, more distal, airways the mucous is found in ‘islands’, and a mucous layer is absent in small bronchioles and beyond. Mucous is composed mostly of glycoproteins called mucins,6,7 which determine the visco-elastic and other properties of the mucous. Mucin is released by rapid (<150 ms) exocytosis from the mucous secreting goblet cells in response to a range of stimuli including direct chemical irritation, inflammatory cytokines8 and neuronal stimulation, predominantly by cholinergic nerves.9,10 Mucins have a core composed of glycoprotein subunits joined by disulphide bonds and their length may extend up to 6 μm. The core is 80% glycosylated with side chains attached via O-glycosidic bonds. Almost all terminate in sialic acid and possess micro-organism binding sites. Mucous plays a vital role in pathogen entrapment and removal, and also has a variety of antimicrobial actions (see below).
Ciliary function.11,12 The mucous layer is propelled cephalad by the ciliated epithelial cells (Figure 12.1) at an average rate of 4 mm.min−1, to be removed by expectoration or swallowing on reaching the larynx and pharynx. The cilia beat mostly within the low-viscosity periciliary layer of airway lining fluid, with the cilia tips intermittently gripping the underside of the mucous layer, so propelling the mucous layer along the airway wall.

(Kindly reproduced by permission of Dr P. K. Jeffery, Imperial College School of Science, Technology and Medicine, London and the publishers of Respiratory Medicine.13)
Cilial beat frequency is 12–14 beats per second and can be affected by pollutants, smoke, anaesthetic agents and infection. Two phases occur for each beat (Figure 12.2). First is the recovery stroke which occupies 75% of the time of each cycle and involves a slow bowing movement away from the resting position by a sideways action of the cilium. There then follows the effective stroke in which the cilium extends to its full height, gripping the mucous layer above with claws on its tip, before moving forward in a plane perpendicular to the cell below and returning to its resting position. Adjacent cilia somehow coordinate their strokes to produce waves of activity that move the mucous layer along, probably by a physical effect of cilia stimulating adjacent cilia during the sideways sweep of the recovery stroke.
Periciliary layer. For this propulsion system to work effectively, it is crucial that the depth of the periciliary fluid layer be closely controlled,4,5 particularly considering the increasing amount of mucus that will occur each time two smaller airways converge into one larger airway. The depth of both layers of the airway lining fluid is controlled by changes in the volume of secretions and the speed of their re-absorption. If the periciliary layer reduces in depth, the gel layer will compensate for this by donating liquid to the periciliary layer to maintain the correct depth of fluid, an effect probably mediated by simple osmotic gradients between the two layers. The mucous layer may donate fluid to the periciliary layer until its volume is diminished by 70%. The reverse happens as the mucus converges on the larger airways, with the mucous layer absorbing excess periciliary water. The volume of periciliary fluid is therefore effectively determined by its salt concentration, which is in turn controlled by active ion transport on the surface of the epithelial cells. The ion channels responsible for active control are amiloride sensitive Na+ and Cl− channels, the latter better known as the cystic fibrosis transmembrane regulator (CFTR) protein. CFTR is likely to be partially active at rest but is stimulated when Na+ channels are inhibited. The factors responsible for this critical system are unknown, though adenosine diphosphate release in response to cyclical airway movements may be involved.14
In patients with cystic fibrosis an inherited defect of CFTR leads to dysfunction in the regulation of airway lining fluid homeostasis, with severe consequences (Chapter 28).
Humidification. The airway lining fluid acts as a heat and moisture exchanger to humidify and warm inspired gas. During inspiration, relatively cool, dry air causes evaporation of surface water and cooling of the airway lining, then on expiration moisture condenses on the surface of the mucous and warming occurs. Thus only about one half of the heat and moisture needed to condition (fully warm and saturate) each breath is lost to the atmosphere. With quiet nasal breathing, air is conditioned before reaching the trachea, but as ventilation increases smaller airways are recruited until at minute volumes of over 50 l.min−1 airways of 1 mm diameter are involved in humidification.
Inhaled Particles
Defence against Inhaled Pathogens
Chemical inactivation of pathogens.15 Airway lining fluid is more than a simple transport mechanism for impacted micro-organisms. Some smaller particles will penetrate far into the bronchial tree and take some time to be transported out of the respiratory tract. To prevent these organisms from causing damage during this time, the airway lining fluid contains multiple systems for directly killing pathogens. Surfactant, in addition to its role in reducing lung compliance (page 30), also acts as part of the innate defences in the lung.16 Surfactant protein A is the most active surfactant protein in pulmonary defence, its actions including stimulating macrophage migration, the production of reactive oxygen species, and the synthesis of immunoglobulins and cytokines. Both surfactant protein A and D are directly bactericidal,17 with activity against many common pulmonary pathogens. Lysozyme is also present in airway lining fluid. This enzyme, secreted by neutrophils, is capable of destroying microbial cell walls causing bacterial lysis, particularly for Gram-positive bacteria.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree