6 Non-Balloon Coronary Interventional Techniques and Devices Rotational Atherectomy, Thrombectomy, Cutting Balloons, and Embolic Protection Devices
Rotational Atherectomy
Indications and Contraindications
1. The treatment of fibrocalcific plaques with balloon angioplasty often results in incomplete lesion dilation, which subsequently increases the risk of restenosis or acute/subacute stent thrombosis. Aggressive attempts to dilate resistant lesions with either high-pressure inflations or the use of noncompliant balloons carries the risk of vessel wall injury (i.e., dissection or perforation).
2. Non-dilatable lesions often inhibit the passage of balloons and stents, thereby limiting options for definitive revascularization. In these clinical situations, initial plaque debulking with RA may improve initial procedural success by improving arterial compliance, thereby facilitating more uniform and symmetric stent deployment while potentially allowing for increases in postprocedure minimal lumen diameter. Finally, while treating bulky lesions at or near a large branch vessel, preemptive use of RA may help minimize plaque shifting (i.e., the “snow-plowing” phenomenon) and alleviate the need for subsequent side branch intervention.
Although RA may assist in the delivery and deployment of interventional equipment (i.e., balloons and stents), there are specific clinical situations where its use should either be used with great caution or avoided altogether (Table 6-1).
Table 6-1 Indications and Contraindications to Rotational Atherectomy
| Indicated | High-Risk | Contraindicated |
|---|---|---|
LV, left ventricular; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Equipment
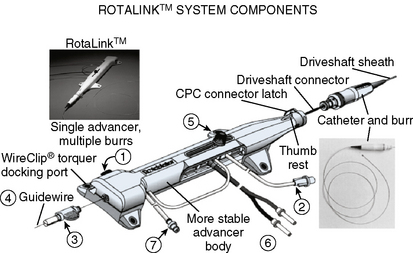
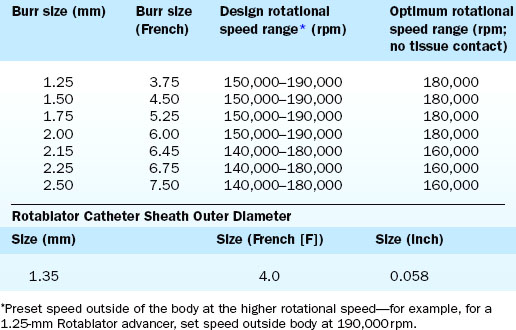
The Rotablator Rotational Atherectomy System (Boston Scientific Corporation, Natick, MA) is the only commercially available RA system (Figs. 6-1 and 6-2). The system consists of a nickel-plated brass elliptical burr (available in sizes of 1.25–2.5 mm in diameter) that is coated on its leading edge with diamonds that are 20 to 30 microns in diameter. The burr is attached to a long, flexible driveshaft that is inserted through a guide catheter (6–10 F; see Table 6-2) over a 0.009-inch stainless steel guidewire (i.e., RotaWire). The RotaWire is available in both extra-support and floppy grades of stiffness. Whereas the floppy RotaWire is used in the majority of cases, the extra-support version may assist in advancing the device to very distal lesions. Finally, the Rotablator driveshaft itself is contained in a 4.3 F Teflon sheath and is connected to a turbine driven by compressed nitrogen gas. A continuous infusion of pressurized emulsifier solution (i.e., Rotaglide) with saline is infused through the drive shaft to aid lubrication and heat dissipation.
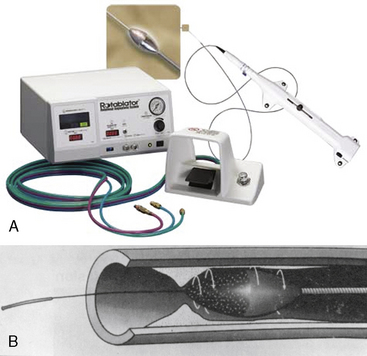
Figure 6-1 A, Rotablator Rotational Atherectomy System. B, Diagram of the rotational atherectomy burr.
(Courtesy of Boston Scientific Corp., Natick, MA.)
Table 6-2 Recommended Guide Catheter Sizes for Use With the Coronary Rotablator
| Rotablator Burr Size (mm) | Recommended Guide Catheter Internal Diameter (inch) | Guide Size (French [F])* |
|---|---|---|
| 1.25 | 0.053 | 6–8 |
| 1.50 | 0.063 | 6–8 |
| 1.75 | 0.073 | 6–8 |
| 2.00 | 0.083 | 7–9 |
| 2.15 | 0.089 | 7–9 |
| 2.25 | 0.093 | 7–9 |
| 2.50 | 0.102 | 9–10 |
* For a given size of catheter, the inside diameter varies from manufacturer to manufacturer. French sizes assume thin-wall (high-volume flow) catheters with side holes.
Technique and Technical Tips
Crossing of the lesion can be performed either with the RotaWire or with a standard 0.014-inch guidewire that can subsequently be exchanged for a RotaWire using either a tracking catheter or an over-the-wire balloon system. Although the RotaWire size (0.009-inch) often makes crossing lesions challenging, in cases where exceptionally severe lesions preclude the passage of either a tracking catheter or over-the-wire balloon catheter, direct wiring with the RotaWire may in fact be necessary. The infusion port on the drive shaft is connected to a pressurized bag of saline and lubricant mixture (i.e., Rotaglide—egg yolk/olive oil/EDTA mixture). A combination of verapamil (10 mg/L), nitroglycerin (4 mg/L), and heparin (2000 U/L) can also be added to the saline flush in order to minimize vessel spasm during RA. After loading onto the RotaWire, the burr’s speed is tested (i.e., platforming) prior to introduction into the guide catheter. The burr speed during platforming should range from 160,000 to 180,000 rpm, depending on the burr size (Table 6-3). Following successful platforming, the burr is advanced through the guide catheter and is positioned immediately proximal to the lesion. Advancement of the burr through the guide catheter around the aortic arch often requires the operator tasked with securing the back end of the RotaWire to provide additional back tension to both facilitate burr advancement and limit acquired tension. Although many operators will transiently activate the system inside the guide to further alleviate acquired tension within the system, activating the system in the vessel proximal to the lesion accomplishes the same goal of preventing the burr from “leaping forward” during the initial RA pass. Direct intracoronary administration of vasodilators prior to system activation can be performed at this time to combat coronary spasm potentially instigated by RA.
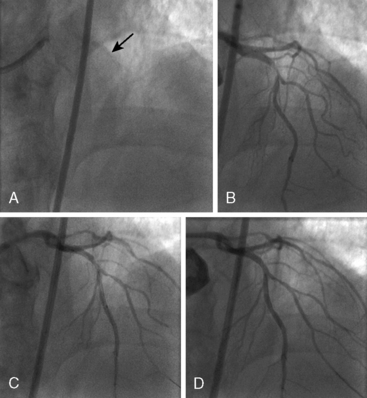
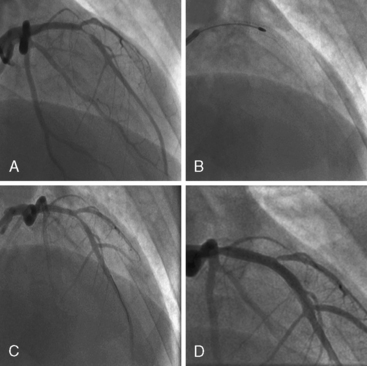
Of note, in particularly severely narrowed lesions with heavy calcium, the RotaWire may retract proximally during burr advancement. By ensuring coaxial guide catheter alignment and applying gentle forward pressure on the guide catheter, distal wire position can be maintained. Finally, if excessive decelerations (i.e., > 5000 rpm decrease) repeatedly occur during RA, it is recommended to downsize to a smaller burr. Figures 6-3 and 6-4 are case examples demonstrating the use of RA in various clinical settings.
No Reflow or Slow Reflow After Rotablator Ablation
No reflow or slow reflow is the occurrence of no blood flow (no reflow) or blood flow reduced by one angiographic thrombolysis in myocardial infarction (TIMI) study flow grade (slow reflow) in the treated artery despite the fact that the treated segment is patent. No reflow or slow reflow is believed to occur because of the transient increase in blood viscosity due to the presence of microparticles or vasospasm at the level of the distal microvasculature. No reflow or slow reflow has been observed in 6% to 7% of patients undergoing PTCA (see Fig. 6-2). No reflow and slow reflow can be minimized by the following actions:
2. Using a stepped burr approach (smaller, then larger) in long or calcified lesions
3. Using a “pecking” motion at the plaque to avoid blocking the arterial lumen
4. Maintaining maximum blood flow by repeated flushing with saline (bolus of 10–30 mL)
5. Using guide catheters with side holes
6. Maintaining the left ventricular filling pressures (and mean arterial pressure) by increasing the patient’s volume status appropriately
When performed in a controlled setting by an experienced operator, RA offers a safe and effective means of debulking plaque and preparing lesions for stent implantation. Additional technical tips on performing safe and successful RA are listed in Table 6-4.
Table 6-4 Technical Notes and Tips on Performing Rotational Atherectomy