(1)
Laboratory of Genetics and Molecular Cardiology, Heart Institute, São Paulo, SP, Brazil
(2)
Federal University of São Paulo Medical School, São Paulo, SP, Brazil
(3)
Department of Cardiac Catheterization and Interventional Cardiology, Hospital do Coração (HCor), São Paulo, SP, Brazil
(4)
Catheterization and Interventional Cardiology, Heart Institute (InCor), São Paulo, SP, Brazil
(5)
University of São Paulo, São Paulo, SP, Brazil
Abstract
Despite the indisputable advances in medical treatment and revascularization procedures (percutaneous and surgical), many patients present debilitating symptoms related to myocardial ischemia which cannot be controlled by a combination of antianginal drugs due to progression of disease with arterial occlusion and diffuse involvement of previous grafts or post-angioplasty restenosis, preventing new attempts of myocardial revascularization. This condition is defined as refractory angina, which greatly impairs the quality of life of the affected. Recently, new therapeutic strategies are being either developed or already applied for the treatment of patients with refractory angina, including gene therapy, stem cell therapy, transmyocardial laser revascularization, enhanced external counterpulsation, spinal cord stimulation, and extracorporeal shockwave myocardial revascularization. However, many of the above techniques are still surrounded by a shadow of controversy, conflicting results between the basic science and clinical application, mixed feelings from the scientific community regarding their usefulness (beyond the placebo effect), and the appropriateness of the conducted clinical trials in which they have been tested. Common challenges in the field are, for example, the fact that there is no experimental model that exactly mimics the condition seen in patients with diffuse CAD; many promising new therapies, i.e. gene therapy, have succeeded in animal models of myocardial ischemia only to fail when rigorously tested in double-blind, placebo controlled trials. Finally, the scientific community should truly be committed regarding the rigor with which data are obtained and presented, so that science may steadily advance towards finding better, proven treatment options for patients with refractory angina.
Keywords
AnginaTreatmentGene therapyStem cell therapyLaserShockwaveCounterpulsationNeurostimulationIntroduction
Cardiovascular disease (CVD) is the leading cause of death worldwide. The World Health Organization [1] estimates that CVD alone was responsible for approximately 17.5 million deaths in 2012 (or 31.4 % of all deaths in that year). CVD deaths are mainly due to ischemic heart disease (7.3 million deaths) or stroke (6.7 million deaths).
One of the most common manifestations associated with ischemic heart disease (IHD) is stable coronary artery disease (CAD), which can be translated clinically by chest discomfort (or equivalent) evoked by different levels of physical activity depending on the extent of the disease. In the United States, approximately 7.8 million people live with the diagnosis of angina pectoris [2].
Despite the indisputable advances in medical treatment and revascularization procedures (percutaneous and surgical), many patients will present debilitating symptoms related to myocardial ischemia which cannot be controlled by a combination of antianginal drugs due to progression of disease with arterial occlusion and diffuse involvement of previous grafts or post-angioplasty restenosis, preventing new attempts of myocardial revascularization. This condition is defined as refractory angina [3]. The estimated annual incidence of patients with refractory angina is between 50,000 and 200,000 new cases in the United States [4] and between 30,000 and 100,000 in Europe [5]. Currently, between 600,000 and 1.8 million individuals are living with refractory angina in the United States [6].
The hallmark of this condition is the great impairment of quality of life [7, 8]. Their goal is to be able to perform any physical activity (no matter how trivial it seems like walking a few meters or even bathing) without anginal pain. Some patients are frequently awakened during the night by angina. Presently, all major Cardiology Societies (American Heart Association and American College of Cardiology [9], Canadian Cardiovascular Society [10] and the European Society of Cardiology [5]) acknowledge the need to seek new therapeutic strategies for this growing population of patients in whom maximally tolerated conventional treatment has failed. For these patients, the primary goal of treatment is to improve quality of life, to increase exercise tolerance, and to decrease the need for hospitalization and diagnostic or therapeutic procedures. In this chapter, we will briefly discuss the main non-pharmacological therapeutic strategies being either developed or already applied for the treatment of patients with refractory angina (Table 11.1) and consider any unrvesolved or controversial areas in therapy.
Table 11.1
New therapeutic options for patients with refractory angina
Therapy | Current status for clinical use (class of recommendation/level of evidence) |
|---|---|
Gene therapy | Investigational |
Stem cell therapy | Investigational |
Transmyocardial laser revascularization | Approved (IIb/B)a |
Enhanced external counter-pulsation | Approved (IIb/B)a (IIa/B)b |
Spinal cord stimulation | Approved (IIb/C)a (IIb/B)b |
Extracorporeal shockwave myocardial revascularization | Approved in a few countries in Europe and Asia |
Investigational in the USA |
It is important to note, however, that many of the above techniques are still surrounded by a shadow of controversy, conflicting results between the basic science and clinical application, mixed feelings from the scientific community regarding their usefulness (beyond the placebo effect), and the appropriateness of the conducted clinical trials in which they have been tested. Table 11.2 shows a few examples of the controversial issues to be explored in the corresponding sessions in this Chapter.
Table 11.2
Controversial issues in therapeutic options for patients with refractory angina
Therapy | Controversy/unresolved issues |
|---|---|
Gene therapy | Conflicting results between experimental models (success) and clinical application (disappointment) |
Moved to fast from bench to bedside (!) | |
Placebo effect (?) | |
Stem cell therapy | Conflicting results between experimental models (success) and clinical application (failure or modest benefit) |
Moved to fast from bench to bedside (!) | |
Placebo effect (?) | |
Unanswered “burning” questions: best cell? Dosage? Route for delivery? Which patient? Long-term safety profile? | |
The “miracle cure” for almost every disease known to man (false advertisement without robust scientific support) | |
Transmyocardial laser revascularization | Placebo effect (?) |
Class IIb/B in the USA versus a class III in Europe | |
Enhanced external counter-pulsation | Reduction in MACE (?) |
Spinal cord stimulation | Reduction in MACE (?) |
Mechanism of action (?) | |
Extracorporeal shockwave myocardial revascularization | Placebo effect (?) |
Lack of randomized, double-blind, placebo-controlled, properly sized trial |
Gene Therapy
Gene therapy can be defined as a medical intervention for transferring genetic material to somatic cells in vivo, allowing the in situ expression of the transferred gene [11] with therapeutic effect. Administration of therapeutic genes requires the use of a vehicle, called a vector, capable of carrying the gene of interest and guiding it to the target cell, thereby facilitating the transfer of genetic material into somatic cells [12] (Fig. 11.1).
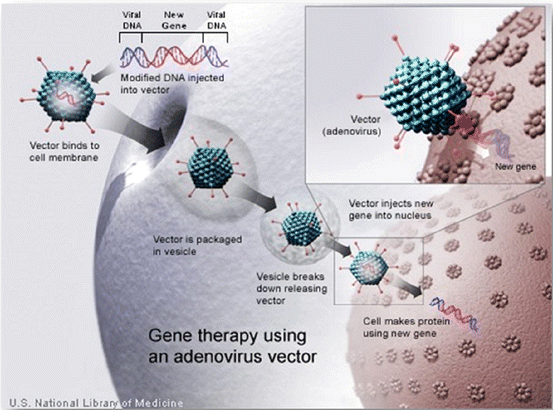

Fig. 11.1
Gene therapy using a modified virus as a vector for gene transfer into somatic cells (Source: United States National Library of Medicine)
The accumulation of knowledge about vascular growth and angiogenic cytokines and the parallel development of more efficient vectors allowed for testing the hypothesis that gene transfer of growth factors could mitigate the damage from myocardial ischemia by stimulating vascular growth, a strategy known as therapeutic angiogenesis [12].
From the late 1990s, many researchers including Losordo et al. [13], Symes et al. [14], Rosengart et al. [15] and others reported the results of the VEGF165 gene transfer by direct intramyocardial injection in patients with refractory angina. During follow-up, they were able to document a significant reduction in the number of angina attacks, a significant decrease in the number of hypoperfused myocardial segments, and an increased Rentrop score (number of collateral vessels) in all patients. No procedure-related adverse effects were observed.
Later on, the AGENT (Angiogenic Gene Therapy) trial [16], the first multicenter study to include 79 patients with symptomatic CAD to receive one of five escalating doses of viral vector encoding FGF4 or placebo, was published. Although the analysis of the overall therapeutic effectiveness based on the exercise test did not show any differences between groups, analysis of the subgroup with greater initial functional impairment showed a significant increase in exercise tolerance. Subsequent studies like the AGENT-3 and -4 [17] trials involving more than 500 patients in several countries did not replicate the results originally obtained regarding better exercise tolerance after administration of FGF4 in patients with stable angina and were, therefore, prematurely interrupted. Similar results were also obtained in the VIVA trial (Vascular Endothelial Growth Factor in Ischemia for Vascular Angiogenesis) [18]. In this trial, clinical evaluation performed at 120 days after treatment showed that the group receiving the highest dose of VEGF had a significant reduction in angina (functional class improvement) with only a favorable trend towards better exercise performance. Because of the lack of consistent, replicable data in terms of efficacy in controlled randomized clinical trials, much of the initial interest in gene therapy for the treatment of patients with refractory angina has faded away.
Cell Therapy
The therapeutic potential of transplantation of stem cells and/or progenitor cells has been explored experimentally for over a decade aiming to induce the growth of new blood vessels (angiogenesis) [19] and/or to regenerate cardiomyocytes after myocardial infarction [20].
Motivated by the initial success obtained in experimental models of myocardial ischemia, the first results of cell therapy applied to patients with CAD were reported in the last decade. Assmus et al. [22] transplanted bone marrow-derived or peripheral blood progenitor cells by means of intracoronary infusion in patients after acute MI. After 4 months, treated patients had improved the left ventricular ejection fraction and the regional wall motion in the infarct area was associated with a lower end-systolic volume and increased coronary flow reserve in the culprit, treated coronary artery was noted. No adverse events were observed.
The use of adult bone marrow-derived cells for treating severe CAD associated with heart failure was proposed by Perin et al. [23]. Fourteen patients underwent trans endocardial injection guided by electromechanical mapping in ischemic but viable areas (hibernating myocardium). The authors showed that, after 4 months, there was an improvement in functional class, significant reduction in perfusion defects assessed by SPECT, and an increase in ejection fraction from 20 to 29 % in treated patients.
Stamm et al. [24] proposed the use of intramyocardial injection of bone marrow-derived stem cells combined with CABG in 6 post-MI patients. Functional assessment revealed an increase in LV global motility (in 4 out of 6 patients) and increased perfusion in the infarct area (in 5 out of 6 patients). Gowdak et al. [25] tested a similar strategy for the treatment of patients with severe and diffuse CAD, refractory to medical therapy and not amenable to complete surgical revascularization strategy because of the extent of the disease. In 21 patients, autologous progenitor hematopoietic cells were injected during CABG in those areas previously identified as viable and ischemic. No adverse events related to the procedure were noted [26]. There was an increase in myocardial perfusion in the injected segments, which have not been grafted, along with improved regional contractility. A large randomized, double blind, controlled trial is underway to test the role of cell therapy as adjunctive therapy to incomplete myocardial revascularization in patients with stable angina [27].
The RENEW study, currently underway, will test the safety and efficacy of intramyocardial injection of autologous CD34+ cells in patients with refractory angina unresponsive to optimal medical therapy and who are not candidates for revascularization procedures [28]. Another study recently launched, the IMPACT-CABG [29], will test the safety and efficacy of intramyocardial injection of autologous CD133+ cells in patients undergoing CABG.
More recently, the angiogenic potential of adipose-derived mesenchymal cells began to be explored in patients with ischemic heart disease [30], acute myocardial infarction and heart failure [31]. These clinical trials will document the possibility, if successful, of using this abundant cell source for the treatment of patients with a large spectrum of CVD.
Finally, one of the last cell types to be tested in the treatment of patients with ischemic cardiomyopathy resulted from the identification of resident cardiac stem cells with the potential for myocardial regeneration [32]. Many preclinical studies have demonstrated the efficacy of these cells in the treatment of post-MI left ventricular dysfunction [33, 34]. In the SCIPIO [35] study, cardiac resident stem cells were obtained from the right atrial appendage during surgery for myocardial revascularization. Once isolated, the cells were expanded and infused via intracoronary about 4 months after surgery. Evaluation of cardiac function by magnetic resonance imaging showed a significant increase in LVEF in the treated group from 27.5 % (baseline) to 35.1 % and 41.2 %, 4 and 12 months after infusion of the cells, respectively, as well as a significant decrease in the area of infarction. However exciting these data might sound, caution must be exercised in the interpretation of these studies due to the small numbers of highly selected patients and intra- and inter-observer variability in infarct size measurements. Anatomical and histological examinations of large numbers of patients treated with these cells are necessary to confirm significant generation of myocytes and decreases in infarct size and fibrosis [36]. Moreover, as with any other form of novel therapy, the use of cardiac resident stem cells will have to face the challenge of a double-blind, randomized, placebo controlled clinical trial so that its contribution for myocardial repair can be determined. But even before that, a shadow of uncertainty was already casted on these preliminary data, as we learned that concerns about the integrity of certain data published have led to an internal investigational on the fairness of the study [37].
Transmyocardial Laser Revascularization (TMLR)
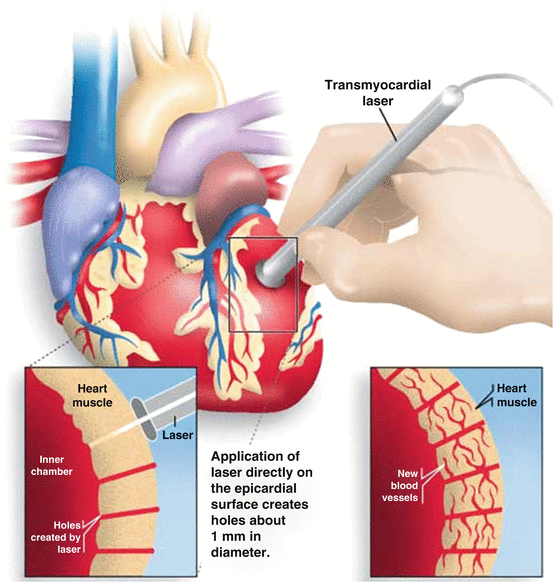
Mirhoseini and Cayton first proposed the use of laser beams for myocardial revascularization in 1981, after a successful experimental study in acute myocardial ischemia model by ligation of the left anterior descendent artery in dogs [38]. The same group published the first report of the clinical use of a CO2 laser as an adjunct strategy to CABG [39]. Transmyocardial laser revascularization (TMLR) is a surgical procedure in which intramyocardial channels (1 mm in diameter) are created through the application of high-energy CO2 laser beams on the heart, without cardiopulmonary bypass, through a left anterolateral thoracotomy. The procedure is based on the premise that myocardial perfusion will increase as blood flows from the myocardial ventricular cavity through the channels created to the ischemic areas (Fig. 11.2).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


