Non-modifiable risk factors:
I. Advanced age
II. Male sex
III. Family history of coronary heart disease
Modifiable risk factors:
I. Hypertension: Elevated systolic and diastolic blood pressure
II. Dyslipidemia: High total cholesterol, high LDL, low HDL
III. Diabetes mellitus
IV. Cigarette smoking
V. Obesity
VI. Physical inactivity
VII. Metabolic syndrome and insulin resistance
Table 1.2
New risk factors for Coronary Heart Disease
Biomarkers: |
I. High sensitivity C-reactive protein (hsCRP) |
II. Albuminuria |
III. Lipoprotein Associated Phospholipase A2 (LP-PLA2) |
IV. Lipid sub-particles: Lipoprotein (a), Apolipoprotein B |
V. Natriuretic peptides (ANP, BNP, NT-proANP, NT-proBNP) |
VI. Cardiac troponin |
Subclinical atherosclerosis imaging techniques and vascular markers: |
I. Coronary artery calcium |
II. Ankle brachial index |
III. Carotid Intima Media Thickness |
IV. Endothelial Dysfunction and peripheral flow mediated dilatation |
V. Measures of arterial stiffness |
There is usually a latency period between exposure to risk factors and development of first CV event. This provides an opportunity to treat high risk patients with lifestyle modifications and medications. Effective evidence-based medical therapies are available to specifically address CV risk factors and various medical and governmental agencies have established therapeutic guidelines. Although implementation of such therapy(s) can reduce clinical events, adherence is generally poor. Medication compliance can be challenging in asymptomatic patients without a prior adverse CV event. Prospectively proven global lifetime risk prediction models may help impress patients and payers of the importance of therapy even in primary prevention.
There has been an emphasis on identifying high risk individuals in whom preventative therapy would be most beneficial. However, the majority of CV events actually occur in patients with low FRS. National Health and Nutrition Examination Survey showed that low risk patients comprise the bulk (85 %) of the general population; they in fact constitute 2/3 s of the overall population risk [6]. Thus, assessment based on traditional risk factors cannot accurately predict CV events in many patients, especially those deemed traditionally at low to intermediate risk. This has led to the search of novel risk factors for CVD.
A new risk factor should be associated with incident events independent of established risk factors. Additionally, it should provide incremental risk assessment. This incremental risk prediction is often documented using Receiver-Operator curve analysis showing the improvement in sensitivity and specificity of a risk prediction model above that achieved using traditional risk factors (Fig. 1.1). This improvement is expressed as an increase in area under the curve (AUC) or the C-statistic. Another measure is its ability to reclassify low to intermediate risk patients to high risk so as to influence aggressiveness of therapy and follow up. In this chapter, we will review some of the new and emerging risk factors and evaluate their association with incident CV disease, scrutinize their discriminatory power and reclassification potential. Unresolved or controversial issues will also be addressed.
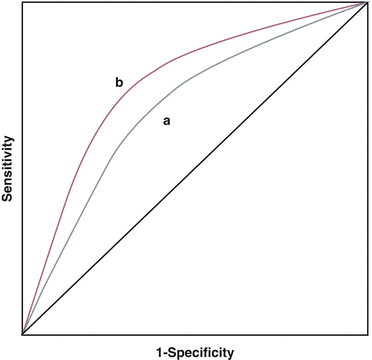

Fig. 1.1
Receiver Operator characteristic curves demonstrating the principle of incremental risk prediction. Area under the curve (a) represents the cardiovascular risk as predicted by established model like Framingham risk score (FRS) that incorporates traditional risk factors. Area under the curve (b) represents risk as predicted by FRS + novel risk factor. Addition of novel risk factor, in this example, moves the curve up and towards the left increasing the sensitivity and specificity of the new model
Biomarkers
Biomarkers can be objectively measured in the body and serve as indicators of normal biological function or pathological processes. A number of biomarkers are associated with CVD. The following is a description of the most prominent CV biomarkers.
High-Sensitivity C-Reactive Protein (hsCRP)
Inflammation is an important player in plaque formation and progression. Chronic inflammatory conditions accelerate atherosclerosis. Inflammatory biomarkers have been evaluated for CV risk prediction with specific attention to their additive value. hsCRP has probably been most widely studied. CRP is an acute phase reactant produced by the liver in response to Interleukin-6 and Interleukin-1 release as a result of infection, trauma or stress. It is a sensitive but non-specific marker of inflammation. CRP is also released at low levels in individuals with atherosclerosis and this can be detected accurately in the circulation. Only hsCRP should be used as a biomarker for use in predicting coronary events.
The role of CRP in atherosclerosis is complex and there is ongoing controversy regarding its mantle as a causative agent versus a marker of vascular inflammation [7]. High levels of CRP are associated with endothelial dysfunction, monocyte chemotaxis and platelet mediated pro-atherogenic effects. CRP attached to oxidized LDL particles can be found in lipid rich plaques. However, this does not establish a cause and effect relationship, particularly since animal and some human research data have failed to establish a crucial role of CRP in the causation of atherosclerosis. Genetic over expression and extraneous injection of CRP in animal models have failed to show increased atherosclerosis. Additionally, gene variants associated with high CRP expression in humans have not shown any significant association with adverse CV events. Thus, CRP might just be a marker for inflammatory changes related to atherosclerosis rather than a key player in the process itself.
Even though causality is contentious, there is substantial evidence that increased CRP is associated with poorer CV outcomes. An analysis of the Multiple Risk Factor Intervention Trial (MRFIT) showed a significant correlation between high hsCRP levels and subsequent CV mortality in healthy but high risk middle age men [8]. The Physicians’ Health Study showed high levels of hsCRP can predict future MI and stroke [9]. The Women’s Health Study found hsCRP to strongly predict CV events compared to LDL [10]. A meta-analysis of 22 studies found levels >3 mg/l were associated with a 60 % increased risk of coronary events versus levels <1 mg/l independent of established risk factors [11].
Does hsCRP add to CV risk prediction over and above traditional risk factors? Studies adding hsCRP to a CV risk prediction model have shown only moderate improvement of AUC. In MESA, coronary calcium score, carotid intima-media thickness, brachial flow-mediated dilatation and ankle brachial index performed better than hsCRP in improving AUC [12]. Other studies failed to show any improvement in risk prediction model at all [13]. The Reynolds risk model incorporates hsCRP and family history to the traditional Framingham risk factors and has shown to add incremental value [14]. However, most of this increment is derived from recalibration of known risk factors with minimal contribution from CRP. HsCRP does reclassify intermediate FRS patients and a low hsCRP value downgrades them to low risk. This reclassification, however, is not as robust as that derived from other testing like coronary calcium scoring.
CRP levels vary among population subgroups. BMI, diet, exercise, infection, smoking and alcohol consumption also influence levels. Interval measurements in the same individual may be significantly different making risk stratification based on a single measurement difficult. Another issue is the threshold of hsCRP to be used in clinical practice. JUPITER (Justification for the Use of Statins in Prevention: An Interventional Trial Evaluating Rosuvastatin) used a cut-off of 2 mg/l for comparing groups [15]. This value is not universally acceptable. In MESA, 3 mg/l performed better than 2 mg/l in predicting events. More importantly, based on representative samples, over 1/2 of the United States population has a CRP level >2 mg/l [16].
HsCRP has increasingly been used to guide statin therapy after JUPITER. JUPITER was a large multi-center trial that randomized 17,802 healthy low to intermediate risk men and women with LDLs <130 mg/dl and hsCRP level >2 mg/l to Rosuvastatin 20 mg daily versus placebo. The trial was stopped early after a median follow up of 1.9 years after a significant reduction (44 %) in combined primary outcome (MI, stroke, revascularization, hospitalization for unstable angina or CV death) was seen in the Rosuvastatin group. Rosuvastatin reduced LDL by 50 % and CRP by 37 %. The greatest reduction in the primary outcome was in patients with a reduction in both LDL and CRP. Some argue that the positive results could be explained by LDL reduction alone. Even in the absence of LDL reduction, the pleiotropic effects of statins with normal or low LDL levels are well established. Thus, while statins lowered CRP, there is no evidence to suggest that their benefits were attributable to their CRP lowering effect. Additionally, JUPITER did not have a low LDL (<130 mg/dl) and CRP (<2 mg/l) arm making it impossible to demonstrate the lack of statin benefit with low CRP. Lastly, some believe that the anti-inflammatory properties of statins are due to their LDL lowering effect. Ongoing trials using anti-inflammatory agents like Methotrexate and Interleukin-1 antagonists in patients with chronic inflammation might shed some light on the specific role of inflammation suppression without lipid lowering.
Due to significant controversy, the enthusiasm for hsCRP in the guidelines has decreased. The 2010 ACCF/AHA guidelines for CV risk assessment in asymptomatic adults gave hsCRP measurement a level IIA recommendation for deciding statin therapy in patients meeting JUPITER criteria [17]. A IIB recommendation was given for men >50 and women >60 years at intermediate CV risk. The 2013 guidelines have lowered all hsCRP recommendations to no higher than IIB [18].
Albuminuria
Microalbuminuria is often considered as poor man’s marker of endothelial dysfunction. Microalbuminuria is generally seen in patients with diabetes mellitus and hypertension. The American Diabetes Association recommends a yearly urinalysis for all diabetic patients. Since 24 h urine collection is cumbersome, early morning samples for albumin-creatinine ratio have been used for diagnosis. Microalbuminuria is a ratio of 30–300 mg/g and macroalbuminuria as >300 mg/g. Multiple cohort studies have linked urinary albumin excretion to CVD risk. A meta-analysis of 26 studies (n = 169,949) showed a positive correlation between albuminuria and incidence of coronary disease [19]. Macroalbuminuria had twice the risk and those with microalbuminuria had a 50 % greater risk of developing CHD irrespective of coexisting hypertension, diabetes and renal function status. Urinary albumin excretion in Framingham and the Cardiovascular Health Study showed minor improvement in the C-statistic [20, 21]. The later study showed risk reclassification with the urinary albumin-creatinine ratio. Subjects with calculated Framingham risk of 5–10 % with urine albumin >30 g/mg had an observed CHD incidence of 20 % versus 6.3 % in the same risk category with lower urine albumin.
The 2010 ACCF/AHA guideline considered microalbuminuria reasonable (class IIA) for CV risk assessment in asymptomatic patients with diabetes or hypertension. For asymptomatic adults without these conditions but still at intermediate risk, the level of recommendation was IIB [17]. The 2013 ACCF/AHA guidelines, citing uncertain contribution of microalbuminuria for risk assessment, have excluded urine albumin measurement in asymptomatic adults [18].
Lipoprotein Associated Phospholipase A2 (LP-PLA2)
Lipoprotein Associated Phospholipase A2 is an enzyme produced by lymphocytes and macrophages that hydrolyzes oxidized phospholipids in LDL. Products of this degradation (lysophosphatidylcholine and oxidized nonesterified fatty acids) are pro-inflammatory and promote atherosclerosis [22]. LP-PLA2 activity is directly related to smoking and LDL and inversely related to HDL levels. Lipid lowering therapy reduces LP-PLA2 levels.
Elevated LP-PLA2 in the plasma and increased LP-PLA2 activity are associated with cardiovascular disease. Meta-analyses of 14 studies (n = 20,549) found an odds ratio of 1.60 (95 % CI 1.36–1.89) for association between LP-PLA2 and CV disease after adjusting for traditional risk factors [23]. Atherosclerosis Risk in Communities (ARIC) study and Rancho Bernardo study showed small improvements in AUC with the addition of Lp-PLA2 to CV risk prediction [24, 25]. However, two different trials evaluating LP-PLA2 inhibitors found no beneficial effect on coronary events and other outcomes [26, 27].
Lipoprotein (a) (LP [a])
Lipoprotein (a) is a lipid sub-particle synthesized in the liver that consists of apolipoprotein B100 linked to apolipoprotein (a). Its exact function in humans is unknown but it has been linked to foam cell formation, inflammation and thrombosis. LP (a) in many studies has been associated with poor CV outcomes. A recent meta-analysis of 24 prospective cohort studies, showed that in patients with 3.5-fold higher LP (a) levels, there was a small increase in CHD (risk ratio 1.13) and stroke (risk ratio 1.10) [28]. There is insufficient data to suggest any incremental risk benefit from measuring LP (a) levels beyond traditional risk factors.
Natriuretic Peptides
Natriuretic peptides (Atrial natriuretic peptide, B-type natriuretic peptide, N-terminal-proatrial natriuretic peptide and N-terminal-pro B-type natriuretic peptide) are released from the myocardium in response to wall stress. Their utility in diagnosis and prognostication in heart failure is well established. Population based studies from North American (including Framingham) and Europe have found higher levels of natriuretic peptides to be associated with a higher incidence of MI, stroke, heart failure, CV death and all-cause death in asymptomatic patients [29–31]. This association may be secondary to underlying left ventricular hypertrophy and subclinical myocardial damage from hypertension or ischemia though this is speculative. The relationship between BNP and future CV events has neither been rigorously tested for incremental increase in the C-statistic when added to traditional risk factors, nor evaluated for risk reclassification.
Troponin
Cardiac troponin is the preferred biomarker to detect myocardial necrosis in acute coronary syndromes. Troponin elevation is also seen in situations of high myocardial demand and non-ischemic conditions like renal disease, heart failure, sepsis and stroke. Methods for troponin detection have become increasingly more sensitive and the new generation ultra-sensitive assays can detect troponins even in the general population. Increased troponin levels with high sensitivity assays may be associated with poor CV outcomes in asymptomatic patients. Participants (aged 54–74 years, n = 9698) in ARIC with elevated cardiac troponin T (≥0.003 μg/L) had a higher incidence of CAD, heart failure and all-cause mortality after adjusting for traditional risk factors, renal function, hsCRP and natriuretic peptides [32]. Younger asymptomatic individuals (aged 30–65 years, n = 3546) from the Dallas Heart study had a similar increase in adjusted all-cause mortality with elevated troponin levels [33]. This raises the possibility of low level myocardial ischemia or stress in these asymptomatic patients. However, this relationship needs further evaluation and validation. It has the same limitations as natriuretic peptides and measuring troponin levels in the general population does not have a present role in clinical practice. The guidelines have not discussed it.
Subclinical Atherosclerosis Imaging Techniques and Vascular Markers
Coronary Artery Calcium
As part of atherosclerosis, calcium deposits in diseased vessel walls. Atherosclerosis progresses with age and the elderly have significantly more calcium in their arteries compared to the young. Coronary artery calcium (CAC) gives an estimate of plaque in the arterial wall. Its non-linear relationship to luminal narrowing (as seen on angiogram) is probably explained by positive remodeling of the vessel. Though calcified plaques are relatively stable, their presence indicates coexisting soft plaque responsible for most acute coronary events. CAC can be detected on 2.5–3 mm thick axial images obtained from non-contrast ECG-gated electron beam or multi detector computed tomography (CT). Based on the area and density of calcium, a calcium score (measured in Agatston units) is obtained and acts as a surrogate marker for global coronary plaque burden [34]. The test can be performed within minutes and is highly sensitive (nearly 100 % for coronary plaque) with virtually no false positive results. However, as previously mentioned, it is not very specific for ‘obstructive’ CAD.
CAC correlates well with future CV events. Pooled data of 27,622 asymptomatic patients from six studies showed incremental rates of MI and CV deaths with increasing CAC score. Subjects with CAC of 0 had a very low (0.4 %) 3–5 year event rate. When compared to this group, the relative risk of having an event with scores of 1–112, 100–400, 400–999 and ≥1000 were 1.9, 4.3, 7.2 and 10.8 respectively [34]. Subsequent studies have shown this relations ship to be consistent. Detrano et al. studied CAC in 6722 men and women from four major racial and ethnic groups (white, black, Hispanic and Chinese) and showed that a doubling of CAC score increased major coronary event risk by 15–35 % and risk of any coronary event by 18–39 % [35]. Predictive values of CAC scores were similar in all racial groups. Addition of CAC to traditional risk prediction models significantly improves AUC. MESA showed an AUC improvement from 0.79 to 0.83 with the addition of CAC (P = 0.006) [36]. This improvement was significantly superior to that seen with hsCRP, carotid intima media thickness or ankle brachial index. CAC also has a reclassification benefit. Intermediate risk patients (with a 10 years CV event rate of 10–20 % as predicted by FRS) with scores of >300 had a 10 years event rate of approximately 28 % [37]. Thus, CAC reclassified these patients to high risk. High CAC scores in asymptomatic patients may motivate some towards healthier lifestyle changes [38, 39] and positively influence aspirin and statin use [40].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


