Although atrial fibrillation (AF) symptom severity is used to guide clinical care, a simple, standardized assessment tool is not available for routine clinical use. We sought to develop and validate a patient-generated score and classification scheme for AF-related symptom severity and burden. Atrial Fibrillation Symptom and Burden, a simple 2-part questionnaire, was designed to assess (1) AF symptom severity using 8 questions to determine how symptoms affect daily life and (2) AF burden using 6 questions to measure AF frequency, duration, and health-care utilization. The resulting score was used to classify patients into 4 classes of symptom and burden severity. Patients were asked to complete the questionnaire, a survey evaluating the questionnaire, and an Short Form-12v2 generic health-related quality-of-life form. Validation of the questionnaire included assessments of its reliability and construct and known groups validity. The strength of interrater agreement between patient-generated and blinded provider–generated classifications of AF symptom severity was also assessed. The survey had good internal consistency (Cronbach α >0.82) and reproducibility (intraclass correlation coefficient = 0.93). There was a good linear correlation with health-related quality-of-life aggregates measured by Pearson correlation coefficient (r = 0.62 and 0.42 vs physical component summary and mental component summary, respectively). Compared with physical and mental component summary scores, the patient-generated symptom severity classification scheme showed robust discrimination between mild and moderate severity (p <0.0001 and p = 0.0009) and between moderate and severe groups (p = 0.0001 and p = 0.012). In conclusion, this simple patient-generated AF classification scheme is robust, internally consistent, reproducible, and highly correlated with standardized quality-of-life measures.
Symptoms of atrial fibrillation (AF) adversely affect quality of life (QOL). Interventions to control rate and rhythm have consistently improved health-related quality of life (HrQOL) in patients with AF. Symptom severity is a well-recognized end point for providers when managing patients with AF. Moreover, a symptom score was recently introduced into the European Society of Cardiology guidelines for the management of AF. However, the clinical community lacks a validated classification system for AF severity. Although initial studies largely lacked validation, in recent years there have been several attempts to design and validate AF-specific questionnaires that can assess symptom severity and its impact on QOL. Because providers and patients consider symptom relief and QOL to be primary goals in the treatment of AF, there is a pressing need for a validated tool to assess AF-specific patient-reported outcome. Our goal was to develop a simple, yet comprehensive, classification scheme for AF symptom severity and burden based on patient perception that integrates both somatic and psychological components. This classification scheme could serve as a clinical and research tool to assess symptom severity and burden and to capture changes resulting from treatment interventions such as drug therapy or catheter ablation.
Methods
The methodologic criteria established by the Food and Drug Administration for the development of a patient-reported outcome instrument served as a framework for the formulation of this instrument. Questions were generated based on the input of senior cardiac electrophysiologists and review of published AF-specific questionnaires in an effort to capture the most representative variables related to the impact of AF on QOL. Given the relation between symptoms of AF and QOL, both domains were incorporated into 1 question. Because side effects of AF therapies may also affect QOL, a question addressing this variable was included. The psychological impact of AF was addressed with 2 questions on AF-related anxiety. Data were gathered on the first 100 patients with AF (paroxysmal and persistent) who completed the pilot draft. A factor analysis showed good load distribution of each variable and an acceptable internal consistency (Cronbach α = 0.76). One of the 2 questions related to anxiety was eliminated as it was considered redundant.
Atrial Fibrillation Symptom and Burden (AFS/B) is a patient-generated classification scheme using scaled responses and composed of 2 separate categories ( Table 1 ; Supplementary Appendix 1 ). Atrial fibrillation symptom (AFS) severity is assessed by a set of 8 questions capturing the effect of symptoms on daily life. Each question has 4 choices and is scored on a 5-point Likert-modified scale giving an AFS score ranging from 0 to 40. Patients were classified prospectively into 4 AFS classes (I, asymptomatic; II, mild; III, moderate; and IV, severe) based on an incremental sum score. Atrial fibrillation burden (AFB) was assessed with 6 questions relating to objective measures of disease and health-care utilization. A subset of burden-related questions addressing frequency and duration of episodes alone was used for scoring, and, based on these 2 variables, patients were classified into 4 AFB classes (A, none; B, minimal; C, moderate; and D, severe burden). The cut points separating each class were determined prospectively ( Supplementary Appendix 2 ).
| Males (n = 173) | Females (n = 61) | p-Value ∗ | |
|---|---|---|---|
| Age | 62.6 ± 11.0 | 66.8 ± 11.0 | 0.0112 |
| AFS score | 10.1 ± 7.5 | 14.3 ± 7.8 | 0.0001 |
| AFB (strict) | 4.4 ± 3.2 | 4.9 ± 2.5 | 0.29 |
| AFB (total) | 9.2 ± 5.2 | 11.6 ± 5.2 | 0.0034 |
| PCS | 47.8 ± 10.0 | 41.0 ± 10.3 | <0.0001 |
| MCS | 52.8 ± 9.0 | 50.0 ± 11.2 | 0.575 |
We enrolled patients with AF visiting 5 different electrophysiologists at 1 center from November 2010 through March 2011. Patients were asked on a first-come basis to complete the AFS/B questionnaire, a survey evaluating the questionnaire, and an Short Form (SF)-12v2 generic HrQOL form. The SF-12v2 is a shorter version of the SF-36v2 HrQOL form, which has been widely used to measure functional health and well-being from the patient’s point of view. At the end of each visit, providers (blinded to patients’ responses) classified symptom severity and burden into 1 of 4 classes for each category. Basic criteria were given to providers to reduce the interrater assessment variability. The provider confirmed that the patient was seen for AF, determined the type of AF (paroxysmal, persistent, and long-standing persistent), and documented each patient’s treatment history. Data on age, gender, pattern of AF, and treatments for AF were gathered. Patients were excluded from the study if they had undergone catheter ablation in the past 2 months or cardioversion and/or major surgery in the last month.
Psychometric analysis of the questionnaire relied on assessment of several features including reliability, construct and known groups validity, and responsiveness. Reliability was assessed by evaluating internal consistency and test-retest reliability. Internal consistency as measured by Cronbach α examined whether different variables intended to measure the same domain were correlated. A test value of 0.70 to 0.90 is considered to represent a strong correlation. Test-retest reliability assesses the stability of responses over time and is measured by intraclass correlation coefficient for which values of >0.7 are considered acceptable. The intraclass correlation coefficient was calculated for AFS mean score, its components, and frequency and duration of episodes in patients with paroxysmal AF who completed the questionnaire at baseline and at least 1 month later. Cases without major interventions and changes in health conditions in the interim period were selected in this analysis.
Convergent validity establishes the validity of a new test; the new measure is correlated with other measures that evaluate the same concept. The generic HrQOL form and SF-36 and its shorter SF-12 version are universally used for this analysis. We assessed the correlation of AFS and AFB scores with physical component summary (PCS) and mental component summary (MCS) scores of the SF-12v2 by calculating Pearson correlation coefficients (r). Correlations with an r of ≥0.4 were considered acceptable. Conversely, divergent validity is demonstrated when items that are believed to measure different domains have low correlations (r <0.4).
The known groups method supports construct validity and evaluates the questionnaire’s ability to discriminate among groups of different symptoms in patients with the same condition. Analysis of variance was used to compare mean scores of PCS between each AFS symptom group (I to IV). Pairwise t tests comparing mean PCS scores of adjacent AFS patient-generated and provider assessment classes were also performed to analyze the “class effect.”
The strength of interrater agreement between patient-generated and blinded provider–generated classifications of AF symptom severity and burden was assessed using the weighted kappa. Responsiveness reflects the ability of an instrument to capture meaningful changes in a patient’s health status over time and more specifically after treatment interventions. This component of the questionnaire will be assessed in a future prospective trial in patients undergoing catheter ablation for AF.
For all statistical tests, a 2-tailed p value of ≤0.05 was considered significant. Analyses were performed using SAS, version 9.3, and MedCalc statistical program.
The study was approved by the Massachusetts General Hospital Institutional Review Boards (Protocol number: 2010-P-002294).
Results
A total of 244 patients were enrolled. Ten patients (4%) were excluded and 10 did not provide sufficient data for SF-12v2 scoring, leaving 224 patients for the main analysis. The main reasons for postenrollment exclusions included failure to complete >50% of forms (4 patients); undergoing recent catheter ablation and completing AFS/B form referring to preablation period (2 patients); and incorrectly completing forms (4 patients). Twelve patients (5%) did not have a provider form completed. The 8 AFS questions had complete data save 1 missing response related to fatigue, and the AFB questionnaire was 97% complete.
Among 218 patients with provider-reported AF type, 119 (55%) had paroxysmal AF, 71 (33%) had persistent AF, and 28 (13%) long-standing persistent AF. Sixty-three patients (28%) had a history of catheter ablation for AF. The mean age was 63.7 ± 11 years, and 74% of subjects were male. An age-related regression analysis noted that as age increased, the AFS score decreased slightly (p = 0.051; Figure 1 ). The age-adjusted mean AFS score was significantly higher for women than men (β = 4.6, p = 0.0001). Women made up 43% of the severe class versus only 11% of the asymptomatic class. Women also reported a significantly higher total burden score than men, which decreased significantly with age (p = 0.030); however, there was no significant age (p = 0.37) or gender effect on the strict burden score (AF frequency plus duration). The effect of age did not vary by gender for either AFS or AFB. The PCS score decreased significantly (p = 0.0013) with age, whereas the increase in MCS score with age was marginally significant (p = 0.0465). Women reported a significantly lower age-adjusted mean PCS or QOL score compared with men and a trend toward a lower mean MCS score ( Table 1 ).
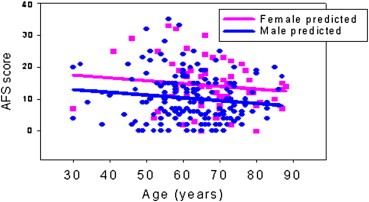
The internal consistency of AFS variables was good (Cronbach α = 0.82). The reproducibility of the questionnaire was assessed for each of the 8 individual symptom elements as well as frequency and duration of paroxysmal AF in 22 test-retest patients. Test-retest reliability as measured by intraclass correlation coefficient was 0.93 for AFS mean scores ( Figure 2 ), ranging from 0.72 to 0.96 for the 8 symptom elements and 0.9 for both frequency and duration.
There was a good linear correlation between patient-generated AFS scores and PCS and a moderate linear correlation with MCS (r = 0.62 and 0.42, respectively; p <0.0001 for both). A higher correlation between related items in both questionnaires (e.g., anxiety vs mental health and sum score of “physical” variables of AFS vs physical role component of SF-12v2) was observed, whereas correlations between unrelated items were lower (e.g., “physical” variables of AFS vs mental component of SF-12v2). The correlation between patient-generated burden score and SF-12 aggregates was low but statistically significant (p value = 0.0002 and 0.021 vs PCS and MCS, respectively). The global health item of the SF-12v2 also correlated better with AFS than AFB scores (r = 0.52 vs 0.20, respectively). Provider-generated AFS classification correlated weakly with HrQOL metric aggregates (r = 0.36 and 0.33 vs PCS and MCS, respectively). A similarly poor correlation was noted between the provider-generated AFB classification and HrQOL metrics. In contrast, the correlation between provider- and patient-generated AFB classifications was strong as was the correlation between provider- and patient-generated AFS classifications ( Table 2 ). The difference in correlations (r) between patient-generated AFS and provider-generated AFS versus PCS scores was significant (p = 0.0048). The overall correlation (r) between AF symptom and AF patient-reported burden score was 0.39 but was higher for paroxysmal versus persistent AF groups (0.59 vs 0.24).
| AFS Score | Provider-AFS | Patient-AFB | PCS | MCS | PR ∗ | MH † | |
|---|---|---|---|---|---|---|---|
| AFS score | 0.73 | 0.39 | −0.62 ‡ | −0.42 | −0.66 | −0.48 | |
| Provider-AFS | 0.73 | −0.36 | −0.34 | ||||
| Patient-AFB | 0.39 | −0.25 | −0.16 | ||||
| Provider-AFB | 0.33 | 0.75 | −0.27 | −0.06 | |||
| AFS physical § | −0.63 | −0.38 | −0.68 | −0.42 | |||
| Anxiety | −0.34 | −0.41 | −0.40 | −0.48 | |||
∗ Physical role (QOL subscale).
† Mental health (QOL subscale).
‡ Negative sign reflects the fact that scales are scored in different directions (higher is better for QOL/SF-12v2 metrics but worse for AFS and AFB).
§ AFS score without anxiety and drug side effects contribution; other abbreviations as in Table 1 .
The results of analysis of variance demonstrated that the patient-generated AFS classification, when tested against PCS and MCS scores, differentiated robustly between mild and moderate severity classes (p <0.0001 and p = 0.0009) and between moderate and severe classes (p = 0.0001 and p = 0.012). There was a nonsignificant trend separating asymptomatic and mild categories ( Table 3 ; Figure 3 ). A t test analysis of the same variables (PCS vs AFS classes) revealed a statistically significant difference between each consecutive group (p = 0.037, p <0.0001, and p = 0.013 for I vs II, II vs III, and III vs IV, respectively).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


