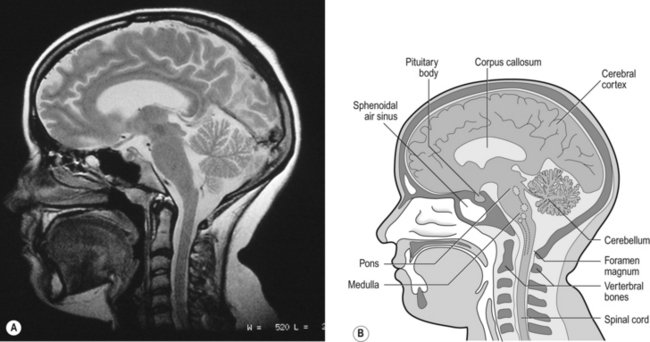
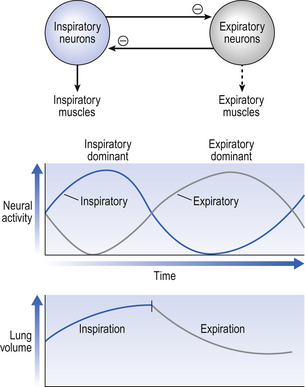
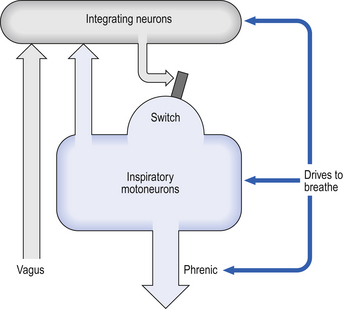
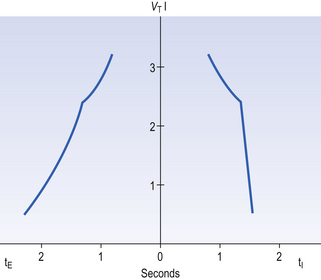
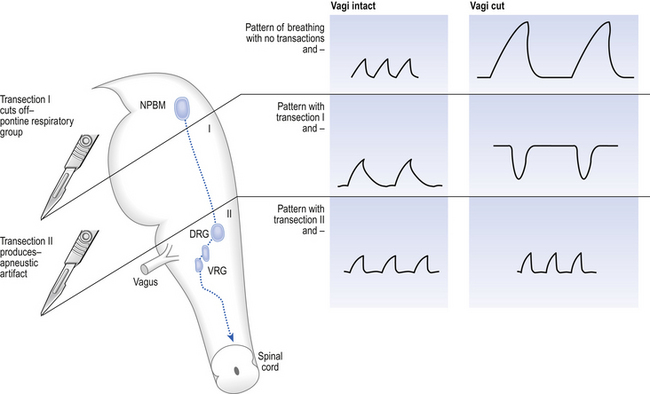
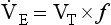10 This chapter is closely related to Chapter 9, which described chemical control of breathing. This division of the subject of control is a semantic one, designed to make learning easier. All chemical control involves neural sensory mechanisms, and it is neural mechanisms that determine and bring about breathing, which in turn plays such an important part in the homeostatic control of the chemical composition of the body. The difference between chemical and neural control is really a matter of time: chemical control, which we have dealt with, takes place over seconds or minutes; neural control reacts in fractions of a second to influence breathing breath by breath. Minute ventilation where VT and f are the tidal volume of each individual breath and the frequency of breathing, respectively. The equation tells us that a particular minute ventilation can be made up of an infinite variety of volumes and frequencies, from high frequencies and small volumes to low frequencies and large volumes. How and why we unconsciously ‘choose’ a particular pattern is the province of neural control of breathing. Economy of energy is an evolutionary advantage, and the pattern of breathing chosen by our bodies is aimed at minimizing the amount of work we have to do to produce a particular minute ventilation. This work is directly related to the force exerted by the respiratory muscles, and it may be that we aim to minimize the tension in our respiratory muscles and/or minimize work. It seems that we get our respiratory systems to ‘resonate’. The resonant frequency for any particular minute ventilation depends on the value of that minute ventilation and the mechanical properties of the lungs (which have been dealt with in Chapters 3 and 4). Breathing originates in the brainstem (Fig. 10.1) which is made up of the medulla (which means marrow, as in bone marrow) and the pons (which means bridge), which connects the medulla to the rest of the brain. The neural basis of breathing within the brainstem is the central pattern generator, whose output to the respiratory muscles is modulated by numerous afferent inputs. Nerve impulses leave the central nervous system via the phrenic and intercostal nerves to bring about breathing by contraction of the respiratory muscles, mainly the diaphragm and intercostals. Other nerves to accessory muscles (e.g. of the larynx) synchronize their contractions with the phases of breathing. The basic pattern of breathing originates in that part of the brainstem which joins the spinal cord to the midbrain and cerebellum, shown in Figure 10.1 and in more detail in Figure 10.5. This region consists of the medulla and pons. If the brain above the medulla is removed (as by Transection II in Figure 10.5) the breathing pattern is remarkably normal. Breathing only ceases when connections between the medulla and spinal cord are cut. (There is some evidence that there are rhythm generators capable of producing breathing movements in the spinal cord itself, but this is a very minor effect occurring under very limited conditions.) The major generator of basic respiratory rhythm is situated in the medulla and is influenced by higher regions of the brain and by activity from receptors in other parts of the body. The idea that oscillating activity in the phrenic nerve could originate in a group of neurons that simply increase and decrease their activity is untenable because there is no reason why such a system should not simply ‘stick’ in the on or off position, which would result in the subject being ‘stuck’ in inspiration or expiration. This argument also applies to the old but persistent idea of two groups of neurons that produce either inspiration or expiration and reciprocally inhibit each other (Fig. 10.2). All plausible models of the central pattern generator start with inspiratory neurons, because to generate a pattern of quiet breathing that is all that is required, expiration in quiet breathing being passive. Most of these models involve some sort of self-limiting negative feedback in the medulla, which operates an ‘off switch’ that limits inspiration (Fig. 10.3). The durations of the two phases of breathing (inspiratory duration, tI, and expiratory duration, tE) are under independent control and so can change independent of each other, or both can change at the same time. Both are influenced by the volume of the lungs. Thus when breathing is accelerated tE is the first to be shortened as VT increases, with tI remaining fairly constant until a threshold is reached, after which it begins to shorten significantly (Fig. 10.4). • All brainstem neurons involved in inspiration are linked and all brainstem neurons involved in expiration are linked by self-exciting connections which synchronize their activity. • On the other hand, there are self-inhibiting connections between all the neurons of the inspiratory group and all the neurons of the expiratory group, which limit the duration of action of each group. • If there is any activity in the expiratory neuron group during eupnoea (quiet breathing) it does not reach a level that activates the expiratory muscles of the abdominal wall, expiration is passive in normal quiet breathing. The relationship between VT, tI and tE shown in Figure 10.4 is disrupted in disease. In chronic obstructive pulmonary disease, for example, overall minute ventilation ( Within this increase in Disconnecting the upper pons from the brainstem (Transection I in Fig. 10.5) removes the effect of the pontine respiratory group (PRG). The neurons that make up this centre are found in and around the nucleus parabranchialis medialis (NPBM). When Transection II (Fig. 10.5) is made, with the vagi cut, breathing becomes slower and deeper. This rate-controlling, volume-limiting effect of the PRG is probably a result of the inspiratory neuron group of the medulla stimulating the PRG during inspiration. When stimulated in this way the PRG, after a short delay, sends inhibitory impulses back to the inspiratory neurons, cutting short their activity (and hence phrenic nerve discharge to the diaphragm) in a classic negative-feedback arrangement. Shortening one breath in this way means that the next breath can start earlier, that is, the frequency of breathing is increased and the volume of breaths reduced. It has been suggested that the PRG evolved as a means of mediating rapid shallow breathing (panting), initiated for thermal or emotional reasons by higher parts of the brain.
NERVOUS CONTROL OF BREATHING
Introduction
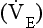 is described by the equation:
is described by the equation:
The rhythm generator
Pattern of breathing in COPD
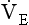 ), which can be thought of as the physical expression of the neural drive to breathe, increases as the disease progresses. This offsets to some extent the decrease in efficiency caused by
), which can be thought of as the physical expression of the neural drive to breathe, increases as the disease progresses. This offsets to some extent the decrease in efficiency caused by 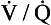 mismatching and changes in lung mechanics.
mismatching and changes in lung mechanics.
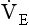 the pattern of breathing also changes. There is initially an increase in VT, but as airways resistance increases as the disease progresses VT decreases below normal. Frequency of breathing increases throughout the progress of the disease. The relationship of inspiratory duration to tidal volume (tI to VT) reflects the drive to breathe. The relationship between inspiratory duration and the total breath duration (tI to tTot) reflects the way a single breath is divided up into the time to fill the lungs (tI) and the time to empty back to the start position (tE). These two phases are of course governed by the mechanical properties of the lungs and airways. Central drive to breathe must increase as airflow limitation progresses, reaching a maximum with respiratory failure. This increased drive effectively increases VT until the increased work of breathing resulting from airflow limitation overpowers it and actually causes a fall in VT. The only way the patient can now increase his minute ventilation is to increase his frequency of breathing. The problem with this strategy is that the expiratory airflow limitation produced by the disease demands that a greater proportion of each breath be devoted to expiration, and the fraction of each breath devoted to inspiration has to be reduced. Air trapping and a subjective sensation of relief when breathing at high volume (which holds the airways open in what has been termed ‘auto-PEEP’) causes the patient with severe COPD to breathe with a rapid shallow pattern at increased lung volumes. This is an inefficient pattern because, in addition to the airflow obstruction, it places the respiratory muscles at a mechanical disadvantage to such an extent that the increased work of breathing can exhaust the patient.
the pattern of breathing also changes. There is initially an increase in VT, but as airways resistance increases as the disease progresses VT decreases below normal. Frequency of breathing increases throughout the progress of the disease. The relationship of inspiratory duration to tidal volume (tI to VT) reflects the drive to breathe. The relationship between inspiratory duration and the total breath duration (tI to tTot) reflects the way a single breath is divided up into the time to fill the lungs (tI) and the time to empty back to the start position (tE). These two phases are of course governed by the mechanical properties of the lungs and airways. Central drive to breathe must increase as airflow limitation progresses, reaching a maximum with respiratory failure. This increased drive effectively increases VT until the increased work of breathing resulting from airflow limitation overpowers it and actually causes a fall in VT. The only way the patient can now increase his minute ventilation is to increase his frequency of breathing. The problem with this strategy is that the expiratory airflow limitation produced by the disease demands that a greater proportion of each breath be devoted to expiration, and the fraction of each breath devoted to inspiration has to be reduced. Air trapping and a subjective sensation of relief when breathing at high volume (which holds the airways open in what has been termed ‘auto-PEEP’) causes the patient with severe COPD to breathe with a rapid shallow pattern at increased lung volumes. This is an inefficient pattern because, in addition to the airflow obstruction, it places the respiratory muscles at a mechanical disadvantage to such an extent that the increased work of breathing can exhaust the patient.
The respiratory ‘centres’
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine