Natriuretic Peptides as Biomarkers to Detect, Risk-Stratify, and Manage Patients with Heart Failure
Susan Isakson
Alan Maisel
Cardiac disease is a leading cause of death around the world, accounting for almost 50% of deaths each year (1). Sixty-one million Americans are living with heart disease (1) and almost 4.7 million of them suffer from symptomatic congestive heart failure (CHF) (2). The cost of managing these patients is significant. An estimated $56 billion per year is spent on diagnosing and treating patients with CHF, 70% of which is due to hospitalization (3). In-hospital mortality and readmission rates of heart failure patients are extremely high (4). Echocardiography, while considered to be the gold standard for the diagnosis of heart failure, is a time-consuming and expensiveprocess, and would delay onset of treatment if routinely relied upon as a major diagnostic tool in the acute setting. As such, there has been considerable interest in the development of reliable biomarkers for use in the diagnosis and treatment of heart failure.
Natriuretic peptides (NPs) have become increasingly important markers for evaluation of the dyspneic patient and treatment of CHF.
While NPs are primarily used as a tool for diagnosing or ruling out acute CHF in the dyspneic patient, recent work suggests a much broader application for NPs. B-type natriuretic peptide (BNP) is not only a useful adjunct for diagnosing and monitoring patients with heart failure (stage C and D), but studies now suggest that BNP provides independent prognostic information with respect to the risk of death or rehospitalization. In addition, BNP may have a role in screening high-risk patients for the presence of underlying cardiac dysfunction (stages A and B) (5). BNP has been shown to have significant diagnostic and prognostic value in patients with heart failure and is becoming increasingly important in the management of both in-patients and out-patients.
Physiology of Natriuretic Peptides
Natriuretic peptides play an important role in the maintenance of fluid balance in patients with CHF. Atrial natriuretic peptide (ANP) and BNP are proteins with 17-amino-acid ring structure that are produced in myocardium. C-type NP (CNP) is another member of the natriuretic peptide family but is secreted primarily from the vascular endothelium, has little influence on fluid status in the setting of heart failure, and has little clinical utility as a biomarker in CHF. ANP and BNP are synthesized in the form of precursors. The precursor of BNP, preproBNP, is cleaved into the signal peptide sequence and proBNP upon release stimulation. ProBNP is further cleaved by a membrane-bound serine protease into the active C-terminal 32-amino-acid BNP molecule and the N-terminal proBNP (NTproBNP) fragment. NPs are released from the ventricles in response to increased pressure inside the heart due to fluid overload and subsequent stretching of the myocytes. ANP is also released in response to stress and volume overload but, unlike BNP, is released from the atria as well as the ventricles (6).
Primary response genes are activated by stretching of the ventricular walls, causing a rapid upregulation of the production of NPs. The body’s response to increased fluid volume and initiation of NP production is rapid; therefore, NP levels accurately reflect the fluid status of the patient. Because NPs are more stable than ANP and because they are primarily secreted by the ventricles, they appear to be more sensitive to left ventricular (LV) dysfunction than are ANP and other neurohormones (7). Unlike ANP, BNP is not released in response to increased activity levels or exercise and is therefore a more accurate indicator of the body’s fluid state. In addition, NPs have a relatively short half-life (22 minutes for BNP and 120 minutes for NTproBNP), making them a valuable tool for monitoring and optimizing the treatment of acutely decompensated heart failure patients (1). NPs have therefore emerged as the marker of choice for diagnosis and management of CHF.
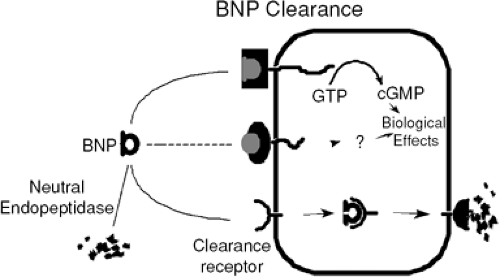
The main physiological function of the natriuretic peptides is to maintain fluid homeostasis in the body. BNP exerts powerful natriuretic, diuretic, and vasodilatory effects on the body. The target sites of BNP and ANP molecules are three natriuretic peptide receptors in the kidneys: NPR-A, NPR-B, and NPR-C. Activated NPR-A and NPR-B catalyze the conversion of guanosine triphosphate to cyclic guanosine monophosphate (cGMP) (Fig. 32-1). In this setting, cGMP is strongly vasodilatory and thereby relieves some of the hemodynamic stress on the heart. When activated, the NP receptors in the kidneys stimulate salt and water excretion and increase the glomerular filtration rate. Reduction of the total fluid volume in the body relieves some of the pressure on the heart and improves hemodynamics. Under normal conditions, the renin-angiotensin-aldosterone system (RAAS) can increase stress on heart and oppose vasodila-tory and diuretic efforts (1). BNP counteracts the sodium-conserving, vasoconstrictive, and volume retention effects of the RAAS. Together, BNP and the RAAS counterbalance each other to regulate fluid volume and arterial pressure. BNP also inhibits the synthesis of catecholamines, angiotensin II, aldosterone, and endothelin 1.
Natriuretic Peptide Assays
The first assay available for use was the Triage Point-of-Care BNP test, approved by the U.S. Food and Drug Administration (FDA) in the fall of 2000. Since that time, a number of assays for both BNP and NTproBNP have been approved and are in clinical use around the world. Table 32-1 is a comparison of the assays and characteristics of BNP and NTproBNP. While it is not within the scope of this chapter to debate the positives and negatives regarding individual molecules and assays, data suggest that both BNP and NTproBNP offer considerable value to the clinician. Thus, it is likely that the final decision as to which biomarker, BNP or NTproBNP, will be used will not necessarily be based on the differences between the two peptides but, rather, on the presence of the pre-existing laboratory equipment necessary to run the assay as well as the perceived need of point-of-care devices versus large laboratory platforms.
One thing can be said with certainty in this regard: The two molecules are not interchangeable. In other words, it would be dangerous for a hospital laboratory to switch from running BNP to NTproBNP, or vice versa, without first making sure the clinicians using the test were aware of
the differences (especially in terms of absolute values), since NTproBNP tends to be about ten-fold more expensive than BNP. In the United States, about 80% to 90% of current NP testing involves BNP (CAP surveys), while in Europe NTproBNP testing is more commonly used. Since BNP testing was the first peptide to be clinically available, experience with its use as well as the development of accepted algorithms have accounted for its increased use in the United States.
the differences (especially in terms of absolute values), since NTproBNP tends to be about ten-fold more expensive than BNP. In the United States, about 80% to 90% of current NP testing involves BNP (CAP surveys), while in Europe NTproBNP testing is more commonly used. Since BNP testing was the first peptide to be clinically available, experience with its use as well as the development of accepted algorithms have accounted for its increased use in the United States.
Table 32-1 BNP Versus NTproBNP Assay Forheart Failure | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Diagnosis of Congestive Heart Failure
Diagnosing acute heart failure is often difficult. Many patients do not exhibit the typical signs and symptoms of heart failure, such as elevated jugular venous pressure (JVP), S3 heart sound, rales, and edema. In addition, many of these symptoms are present in other diseases that can often mimic a CHF exacerbation in patients who present with dyspnea. Incorrect treatment can often worsen the patient’s condition. For example, treatment with sympathomimetic amines and beta-agonists can induce angina and arrythmias in patients with heart failure (8).
Diagnostic tests in the emergency setting, such as routine laboratory tests, chest x-rays, and electrocardiograms, lack the accuracy to make the appropriate diagnosis. Use of echocardiography, the gold standard test for diagnosis of heart failure, is not always practical in the emergency setting because of the duration of the study and the need for a sonographer to perform the test. In addition, it may be very difficult for the patient to remain still long enough to obtain accurate results. In order to avoid the increased morbidity and mortality that parallels delayed and incorrect diagnoses, it is necessary to have a tool with which to differentiate these diseases and make a rapid and accurate diagnosis.
Several studies have established the role of BNP in the clinical diagnosis of CHF. In a study by Dao et al., 250 patients presenting to the emergency department (ED) with dyspnea were evaluated using BNP values (9). Physicians were blinded to the BNP values and were asked to assess the probability that the patients’ dyspnea was caused by an acute CHF exacerbation. BNP values were shown to be the strongest predictors of acute heart failure, being both sensitive to and specific for diagnosing CHF. The mean BNP level in patients found to have CHF was higher than in patients without CHF (1,076 pg/mL versus 38 pg/mL, p <0.001).
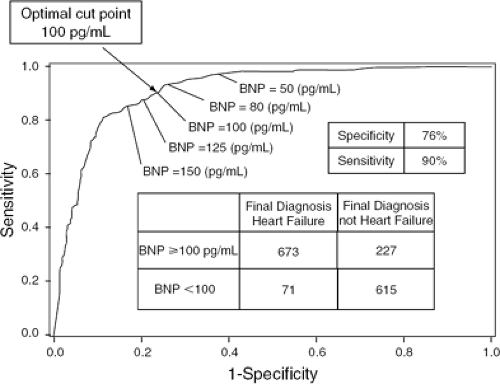
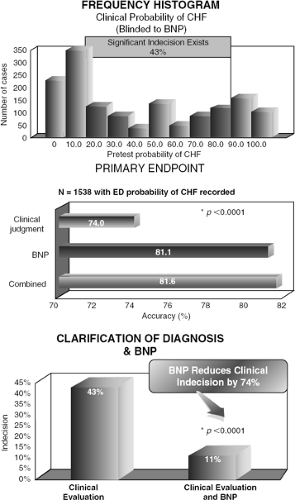
These findings provided a foundation for the Breathing Not Properly multinational study, a prospective study that used BNP levels to evaluate the causes of dyspnea. In this study of 1,586 emergency room patients with acute dyspnea, BNP levels were measured upon arrival. Physicians were asked to assess the probability of heart failure. Later, two cardiologists blinded to BNP values were given the clinical data to review and produce a gold standard diagnosis for each patient. BNP levels were found to be more accurate predictors of CHF than any history, physical findings, or lab values (5). A BNP cut-off value of 100 pg/mL had a sensitivity of 90% and a specificity of 76% for differentiating CHF from other causes of dyspnea, and a cut-off level of 50 pg/mL had a negative predictive value of 96% (Fig. 32-2) (5). A BNP level of >230 pg/mL was associated with a relative risk of 7.0 for a CHF-related event in the absence of a correct diagnosis by the physician (5). Physicians in the ED had a 43% rate of indecision when trying to make a diagnosis in dyspneic patients. BNP levels, had they been available to clinicians, would have reduced the rate of indecision to 11%. Incorporation of BNP into the clinical evaluation of the dyspneic patient was found to increase the absolute diagnostic accuracy by 10% (Fig. 32-3) (10).
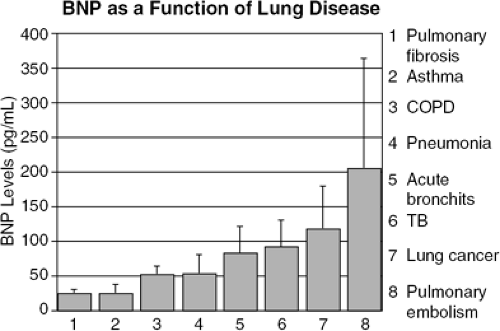
Differentiation between causes of dyspnea is a great challenge faced by ED physicians. It is often very difficult to evaluate whether dyspnea is due to pulmonary disease or CHE. In a study of 321 patients with dyspnea who presented to the ED, BNP distinguished between heart failure (mean BNP 759 ± 798 pg/mL) from pulmonary disease (61 ± 10 pg/mL) and other clinical presentations with a high specificity and sensitivity. The mean BNP values of patients with different pulmonary diseases are shown in Figure 32-4. Moreover, when patients who had a history of heart failure but whose dyspnea was due to chronic obstructive pulmonary disease (COPD) (mean BNP 47 ± 23 pg/mL) were compared with patients who had a history of COPD but whose dyspnea was due to heart failure (731 ± 764 pg/mL), a BNP value of 94 pg/mL yielded a sensitivity and specificity of 86% and 98%, respectively, and differentiated heart failure from lung disease with an accuracy of 91% (11). BNP has therefore emerged as a strong diagnostic and prognostic indicator of LV dysfunction and heart failure.
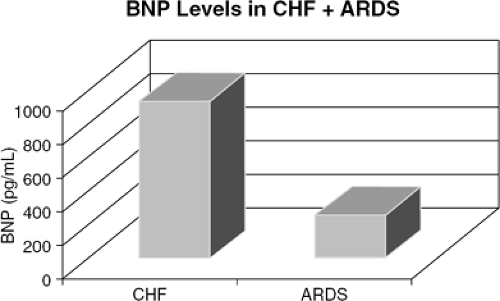
Acute respiratory distress syndrome (ARDS) may also present as dyspnea in the emergency setting and can contribute to the difficulty in making accurate diagnoses in the ED. BNP levels were obtained from 35 patients with ARDS and 42 patients hospitalized with a diagnosis of heart failure. The mean BNP value for those with ARDS was 123 pg/mL, significantly lower than the mean BNP value of 773 pg/mL for patients with CHF (Fig. 32-5). A cut-off
point of 360 pg/mL was determined to have a sensitivity of 90%, specificity of 86%, 89% positive predictive value, and 94% negative predictive value for differentiating between ARDS and CHF (6).
point of 360 pg/mL was determined to have a sensitivity of 90%, specificity of 86%, 89% positive predictive value, and 94% negative predictive value for differentiating between ARDS and CHF (6).
Although NP levels can be elevated in patients with pulmonary disease, NP levels are not usually elevated to the extent that they are in patients with heart failure. Secondary analysis of the Breathing Not Properly multinational study found that measurement of BNP levels can expose underlying CHF in patients with bronchospastic diseases such as asthma or COPD (12). Out of 417 patients studied who had a history of asthma or COPD and no history of CHF, 87 (20.9%) were found to have a final diagnosis of CHF. The mean BNP levels for patients with and without a history of CHF were found to be 587.0 and 108.8 pg/mL, respectively. Therefore, routine BNP testing in patients with a history of asthma or COPD may increase the rate of new diagnosis of heart failure by as much as 20% (12).
Nevertheless, utilization of BNP for the diagnosis CHF is not always straightforward. A gray zone exists in which median levels of BNP are more difficult to interpret and use for making clinical diagnoses. An algorithm developed by Maisel et al. that is now in use at the Veterans Administration Medical Center in San Diego, California and many other institutions is illustrated in Figure 32-6. This algorithm provides guidelines for symptomatic and asymptomatic patients and the likelihood of CHF at different BNP values. For symptomatic patients, a BNP value below 100 pg/mL suggests that the symptoms are not likely due to heart failure. In the range of 100 to 400 pg/mL, the algorithm suggests considering alternative diagnoses such as myocardial infarction, pulmonary embolism, or pneumonia, and clinical correlation is strongly recommended. For patients with existing LV dysfunction, underlying cor pulmonale, or acute pulmonary embolism (PE), a diagnosis of CHF with a BNP in the range of 100 to 400 pg/mL is less likely than for patients without any of these factors (25% likelihood of CHF versus 75% likelihood of CHF). Using this algorithm,
BNP values above 400 pg/mL would be considered highly suggestive of heart failure (13).
BNP values above 400 pg/mL would be considered highly suggestive of heart failure (13).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree