Fig. 12.1
Proteolytic processing of B-type natriuretic peptides (Reproduced from Motiwala and Januzzi [82], with permission from the Nature Publishing Group)
In peripheral organs, biologically active NPs bind to natriuretic peptide receptors (NPR-A, NPR-B, and NPR-C) to initiate a cGMP-dependent signaling cascade. NPR-C and neutral endopeptidase (NEP) work to clear NPs. The physiologic action of ANP and BNP is to counteract the renin-angiotensin-aldosterone system (RAAS) by causing vasodilation, natriuresis, and diuresis. In addition to suppression of RAAS, ANP and BNP contribute to the inhibition of sympathetic overactivation [5]. Thus, natriuretic peptides have an important protective role in heart failure through their complex interactions with neurohormonal pathophysiology and the nervous, renal, cardiac, and vascular organ systems (Chap. 3).
12.3 Diagnosis of Heart Failure
Heart failure is a complex clinical syndrome that is the common end result of many different disease processes. Disorders of the myocardium, pericardium, valves, electrical rhythm, or great vessels can result in structural or functional impairments to the ventricle’s ability to adequately fill with, or eject, blood. As the heart fails, regardless of etiology, decreased cardiac output results in neurohormonal changes that temporarily increase stroke volume. However, over time these neurohormonal changes can lead to volume overload and cardiac remodeling that ultimately results in disease progression. Decreased perfusion from low cardiac output and volume overload from elevated left ventricular pressures lead to the cardinal symptoms of heart failure: dyspnea, fatigue, and fluid retention.
Heart failure is considered a clinical diagnosis, and therefore careful physical exam and history are paramount to making an accurate diagnosis. Additional testing modalities such as chest radiography can also be used to augment a history and physical exam. However, the diagnosis of heart failure can be difficult for several reasons including complex patient population, as well as nonspecific symptomology and exam findings. Careful clinical exam and history can often be complicated in older patients with multiple comorbid conditions. Dyspnea, lower extremity edema, and fatigue can be signs and symptoms of heart failure but are also common in patients with renal disease, chronic lung disease, or obesity. In addition, physical exam findings of increased jugular venous distention or a third heart sound are specific but not sensitive, i.e., the absence does not rule out heart failure. Similarly, chest radiography lacks sensitivity for heart failure, and the specificity of pulmonary edema is limited in the setting of other respiratory illnesses. It is not surprising that diagnostic uncertainty can reach 50 % in patients presenting to the ED with dyspnea [6]. For this reason, there is an important role for biomarkers such as natriuretic peptides to provide adjunctive data to aid in the diagnosis of heart failure (Chap. 1).
12.4 Role of Natriuretic Peptides in the Diagnosis of Heart Failure
Heart failure causes pathologic ventricular wall stretch and results in synthesis and eventual release of the biologically active BNP and inert NT-proBNP [4]. Because the conditions for synthesis and release of NPs are common in heart failure but not in other etiologies of dyspnea, fatigue, or volume overload, the diagnostic value of BNP and NT-proBNP was quickly realized after their discovery. The use of NPs has rapidly been incorporated into clinical practice. The testing of NPs is quick and easily accessible in most emergency, inpatient, and outpatient settings. A potential downside to easy accessibility is that they are often ordered outside of their intended use. A careful understanding of the caveats of NP testing and how to interrupt NPs within the composite of clinical judgment is paramount to maximizing their utility in the diagnosis of heart failure.
The assay for BNP became available prior to NT-proBNP and quickly proved to be useful in establishing and excluding the diagnosis of heart failure. In 2002, the multicenter, multinational Breathing Not Properly trial included 1,586 patients who presented to the ED with dyspnea [7]. BNP values were measured in every patient and blinded to treating physicians. Independent cardiologists, who were also blinded to the BNP results, established the “gold standard” diagnosis. A BNP cutoff of 100 pg/mL was 76 % specific and 90 % sensitive for the diagnosis of heart failure. Compared to components of the history, physical exam, and chest radiography, BNP was the strongest independent predictor of heart failure. In addition, the value of BNP correlated with the severity of heart failure as defined by the New York Heart Association (NYHA) class.
BNP is a quantitative marker of heart failure and is best interpreted along a continuum. When the BNP is low (<100 pg/mL), the clinical presentation is unlikely to be due to heart failure. Using a cutoff BNP value of 50 pg/mL, the negative predictive value of BNP is 96 %. Thus, a low BNP value is useful to exclude the diagnosis of heart failure in the evaluation of dyspnea. In addition to being useful to “rule out” heart failure from the differential diagnosis, BNP is useful in establishing the diagnosis of heart failure. NP values are significantly higher in patients that present with symptoms due to heart failure as compared to other noncardiac etiologies (Fig. 12.2) [8].
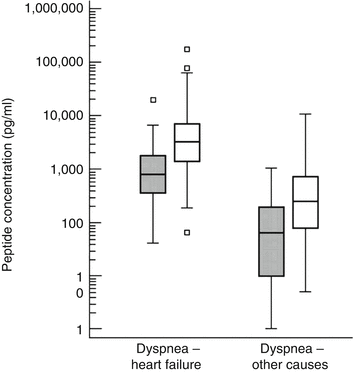

Fig. 12.2
Box and whisker plots of BNP (gray) and NT-proBNP (white) in patients with dyspnea attributable to heart failure as compared to dyspnea attributable to noncardiac etiologies. The central box spans from the lower to the upper quartile, the middle line is the median, and the whiskers extend from the minimum to the maximum concentrates (Reproduced and adapted from reference [8] with permission from the British Cardiovascular Society and BMJ)
The higher the value of BNP, the more likely the diagnosis is heart failure. Using a BNP value of >100 pg/mL, the specificity is 76 %. The ability to “rule in” heart failure increases with the value of BNP. A BNP value >400 pg/mL suggests that the patient’s symptoms are due to heart failure with a specificity that exceeds 90 %. Using a two-cutoff approach (BNP < 100 pg/mL to exclude heart failure and BNP > 400 pg/mL to establish the diagnosis of heart failure), BNP is an accurate biomarker for heart failure. However, as previously noted, this should not be used in isolation. A two-cutoff approach establishes a “gray zone” (BNP values between 100 pg/mL and 400 pg/mL) where further testing and consideration of NP caveats are especially important. Overall, diagnostic accuracy for heart failure is improved when BNP measurement is used in combination with the composite of clinical judgment [9, 10].
Like BNP, NT-proBNP is a quantitative marker and is elevated in heart failure. BNP and NT-proBNP values correlate with each other but are not interchangeable. NT-proBNP values are much higher than BNP, mostly due to a longer half-life. In the ProBNP Investigation of Dyspnea in the ED (PRIDE) study, NT-proBNP was measured in 600 patients with dyspnea. NT-proBNP was highly sensitive and specific for the diagnosis of heart failure. A low NT-proBNP value (<300 pg/mL) can be used to exclude the diagnosis of heart failure, whereas an age-dependent cutoff was used to establish the diagnosis of heart failure. High NT-proBNP, >450, >900, and >1,800 pg/mL in patients aged <50, 50–75, and >75 years old, is highly specific for a diagnosis of heart failure (Fig. 12.3) [11, 12].
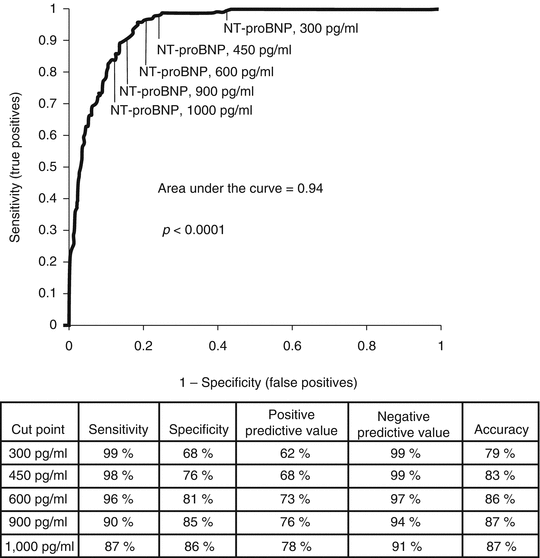

Fig. 12.3
Receiver operating characteristic (ROC) curve for NT-proBNP in evaluation of patients with dyspnea in the ED. Additional statistical information for various cutoffs are presented below the ROC curve (Reproduced from reference [11] with permission from Elsevier)
Both BNP and NT-proBNP are highly sensitive and specific for heart failure but are best utilized in conjunction with interpretation of all available clinical data including comprehensive history, physical exam, and other adjunctive testing. This is especially true when interpreting NP values in the “gray zone” or within the context of comorbid conditions that can affect the value of NPs. When used within the composite of clinical judgment, BNP and NT-proBNP improve diagnostic accuracy, decrease length of stay, and reduce total cost of treatment [10, 13].
12.5 Role of Natriuretic Peptides in Prognosis and Treatment
Heart failure is difficult to diagnose clinically due to nonspecific symptoms and physical exam findings. Similarly, establishing the prognosis and the severity of heart failure at the time of presentation is also difficult using clinical parameters alone. Inpatient heart failure treatment is frequently necessary, and high readmission rates contribute significant cost to the health care system. In addition to being powerful tools in the diagnosis of heart failure, NPs have a role in establishing prognosis and monitoring response to heart failure treatment.
12.5.1 Prognosis
In the REDHOT trial, BNP was predictive of future outcomes in patients who were evaluated for dyspnea and discharged from the ED. Those with a BNP value >400 pg/mL had increased mortality at 90 days compared to patients with BNP < 400 pg/mL [14]. In patients admitted to the hospital, the absolute value of BNP and NT-proBNP and whether these values increased or decreased from the time of admission were predictive of future readmission and mortality [15, 16]. In addition, NPs have prognostic utility in chronic heart failure. In an evaluation of Valsartan Heart Failure Trial (Val-HeFT) comprising 4,300 outpatients with chronic HF, patients with the greatest increase in BNP despite therapy had the highest morbidity and mortality [17]. Used as prognostic markers, NPs may aid in the triage of patients with heart failure and in determining which patients may benefit from closer monitoring or more aggressive therapy.
12.5.2 Treatment
In acute heart failure, NP levels can decline quickly in response to treatment with diuretics and optimization of volume status. In addition, more gradual reductions occur with neurohormonal blockade. Long-term treatment with beta-blockers, aldosterone receptor antagonists, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) lowers NP levels in chronic heart failure. Several studies have evaluated the utility of NPs for medication titration and monitoring response to treatment in the outpatient setting.
In the Systolic Heart Failure Treatment Supported by BNP (STARS-BNP) trial, 220 outpatients with chronic heart failure were randomized to medical care by current guidelines or a treatment goal of decreasing BNP < 100 pg/mL. Patients in the BNP-guided treatment group had less CHF-related mortality and hospitalizations, mainly due to an increase in ACE inhibitor and beta-blocker dosages [18]. Improved mortality at 1 year was also seen in the NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death (BATTLESCARRED) trial. This trial included HF patients with persevered EF and HF patients with reduced EF. Mortality benefit was seen in patients <75 years who had treatment guided by NT-proBNP levels compared to those treated following prescribed clinical guidelines or with usual care [19]. This benefit with biomarker-guided care was not seen in HF patients over age 75 years. Similarly, a lack of mortality benefit with NP-guided therapy in patients >75 years of age was seen in the TIME-CHF study [20].
In the ProBNP Outpatient Tailored Chronic Heart Failure Therapy (PROTECT) study, 151 patients with heart failure with reduced ejection fraction were randomized to standard of care or treatment guided by NT-proBNP with goal NT-proBNP ≤ 1,000 pg/mL. Both patients <75 and ≥75 years of age had decreased cardiovascular events at 1-year post-randomization [21]. This result was driven by decreased hospitalization, where there was >50 % reduction in hospitalizations for acutely decompensated heart failure. There was no statistically significant effect on cardiovascular mortality. In addition to decreasing hospitalizations, the PROTECT study showed significant improvement in quality of life measured by the Minnesota Living with HF Questionnaires and greater improvements in echocardiographic parameters of cardiac structure and function in patients’ treatment with NT-proBNP-guided therapy compared to standard of care [22, 23].
12.6 Caveats to the Use of Natriuretic Peptides
NPs are effective biomarkers in both establishing and excluding the diagnosis of heart failure. However, they are not perfect diagnostic tools and must be used within the context of clinical judgment. An understanding of the caveats of NPs and how to interpret their values within the context of comorbid medical conditions is paramount to the clinical utility of BNP and NT-proBNP. This is especially true when evaluating patients that have NP values that fall into the “gray zone” (BNP values between 100 and 400 pg/mL or NT-proBNP values between 300 and 450–1,800 pg/mL for ages <50, 50–75, >75 years old).
12.6.1 Heart Failure with Preserved Ejection Fraction
Heart failure with preserved ejection fraction (HFpEF), also called diastolic heart failure, is the clinical diagnosis of heart failure in the presence of a normal ejection fraction (EF > 50 %). Patients with HFpEF make up approximately 50 % of all patients with heart failure, and this percentage may be increasing due to change in relative prevalence of various risk factors for heart failure such as obesity [24, 25]. The diagnosis is made clinically through interpretation of signs and symptoms and aided by diagnostic studies, such as an echocardiogram showing the presence of diastolic dysfunction.
BNP and NT-proBNP are sensitive and specific markers for heart failure in patients with preserved ejection fraction. Further analysis from the Breathing Not Properly study showed that BNP was markedly elevated in patients that presented to the ED with dyspnea due to HFpEF as compared to noncardiac etiologies [26]. In addition, the degree of elevation in the value of NPs correlates to the severity of heart failure symptoms by NYHA class and to the severity of objective markers of diastolic dysfunction on echocardiogram [26–28].
Though NP measurement is useful in the diagnosis of HFpEF, NPs are elevated to a lesser extent compared to patients with systolic dysfunction. In the Breathing Not Properly study, mean BNP was 413 and 821 pg/mL in HFpEF and heart failure with reduced EF, respectively [26]. Interpretation of NPs in patients with known preserved systolic function must take into account that NP levels are only moderately elevated in HFpEF compared to higher NP values seen in patients with heart failure with reduced EF. For establishing the diagnosis of HFpEF in the outpatient setting, lower cutoff values for BNP (>200 pg/mL) and NT-proBNP (>300 pg/mL) are suggested. For excluding the diagnosis of heart failure, cutoff values of BNP (<100 pg/mL) and NT-proBNP (<120 pg/mL) are used.
12.6.2 Obesity
Obesity is a known risk factor for cardiovascular events including heart failure [29]. The diagnosis of heart failure in obese patients can be particularly challenging as the interpretation of chest radiography and echocardiography and examination of jugular venous distention can be limited in obese patients. For this reason, the use of NPs for the diagnosis of heart failure in obese patients is appealing. However, an understanding of the relationship between obesity and NPs is essential as there is an inverse association between body mass index (BMI) and NPs in patients with heart failure.
Both BNP and NT-proBNP are lower in obese subjects (BMI >30) as compared to overweight (BMI 25–29.9) and lean (BMI < 25) subjects with heart failure [30–32]. The reason behind the decreased levels is not entirely understood as NPs have a complex relationship with adiposity and lipolysis. Although NP clearance and receptor expression are altered in obesity, NPs are most likely lower in obese patients due to suppression of synthesis and release of NPs from cardiac myocytes [33, 34]. Due to lower BNP levels in obese patients, cutoffs of 170 pg/mL for lean subjects, 110 pg/mL for overweight/obese subjects, and 54 pg/mL in severely/morbidity obese patients are used to maintain a sensitivity of 90 % [35]. Despite lower concentrations of NT-proBNP in overweight and obese patients, NT-proBNP retains it diagnostic capacity across all BMI categories [36]. No adjustment to the previously mentioned NT-proBNP cutoff thresholds is recommended [34]. Overall, due to the inverse relationship between BMI and NPs, it is important to consider obesity when interpreting NP values in the diagnosis of heart failure, especially when these values are in the “gray zone.”
12.6.3 Renal Dysfunction
Heart failure and chronic kidney disease (CKD) commonly coexist. CKD, cardiovascular disease, and heart failure share many of the same risk factors. Approximately 40 % of patients with heart failure have CKD as defined by serum creatinine ≥1.5 mg/dL or estimated glomerular filtration rate (GFR) <60 mL/min/1.7 m2 [37]. Uncontrolled heart failure can cause progression of renal failure through a variety of mechanisms, and, conversely, renal failure can lead to the progression or development of heart failure [38]. In addition, use of efficacious therapies for heart failure, such as ACE inhibitors or aldosterone receptor antagonists (Chaps. 36 and 38), can be restricted by the presence of renal dysfunction [39]. The physiologic and pathologic interactions between the cardiac and renal systems are intricate, and, likewise, the relationship between NPs and renal dysfunction is complex.
BNP and NT-proBNP concentrations are increased in the setting of renal dysfunction. Patients with CKD tend to have increased intravascular volume, elevated pressures, and increased ventricular mass, which can all lead to physiologic elevations in NPs. In addition, decreased renal clearance of NPR-C and NEP may contribute to elevated levels. Because the majority of BNP is not renally cleared, the mechanism of elevated BNP in renal dysfunction is likely multifactorial and not simply decreased passive renal clearance [40]. The Breathing Not Properly study found a correlation between GFR and BNP and suggested a higher cutoff was reasonable for patients with GFR < 60 mL/min/1.7 m2 [41]. Renal dysfunction can possibly explain why some patients have mildly elevated BNP values in the “gray zone” in the absence of heart failure. This further highlights the importance of using BNP within the composite of clinical judgment and not in isolation.
NT-proBNP, though not cleared by NPR-C and NEP, may or may not be more influenced by renal dysfunction than BNP [42]. Due to the association between NT-proBNP and renal dysfunction, the analysis from the PRIDE study suggested a higher cutoff of 1,200 pg/mL. This adjusted cutoff was sensitive and specific for the diagnosis of heart failure in patients with GFR < 60 mL/min/1.7 m2 [43]. Still, NT-proBNP is a strong predictor of 60-day mortality regardless of renal dysfunction [43, 44].
12.6.4 Age and Gender
BNP and NT-proBNP increase with age [11, 45]. This may be due to decreased renal function, but like other endogenous hormones, age may alter production, secretion, or metabolism of NPs [46]. For reference, most young and healthy asymptomatic patients have a very low BNP value, usually less than 20–25 pg/mL [45]. Whereas age stratification is used in establishing the diagnosis of heart failure with NT-proBNP, the same is not suggested for BNP [47].
Healthy females on average have higher NPs than males at all ages [46, 47]. This may be due to increased estrogen or decreased testosterone. However, women with heart failure are more likely to have preserved ejection fraction, which is associated with lower NP values, compared to men with heart failure. Therefore, women with heart failure may have lower NP values than men with heart failure, although this difference has been found to be small [47, 48]. Overall, NT-proBNP and BNP are accurate biomarkers for the diagnosis of heart failure regardless of gender.
12.6.5 Previous Diagnosis of Heart Failure
NPs are elevated in patients with chronic heart failure. In the Breathing Not Properly study, patients with a history of heart failure that presented with dyspnea caused by a noncardiac etiology had an intermediate BNP, compared to those without heart failure and patients with acute exacerbation of heart failure [7]. Patients with chronic heart failure can have an increased BNP during acute exacerbations. In addition, treatment with diuretics, beta-blockers, and ACE inhibitors can decrease BNP over time, however, not always back to a “normal” level. It may be useful to interpret BNP and NT-proBNP in comparison to a “dry” level in patients with chronic heart failure. A “dry” NP, measured when a patient is euvolemic and asymptomatic, can be used as an individualized baseline to judge whether symptoms of dyspnea or fatigue are due to an acute exacerbation of chronic heart failure or due to other, noncardiac, etiologies (Fig. 12.4)
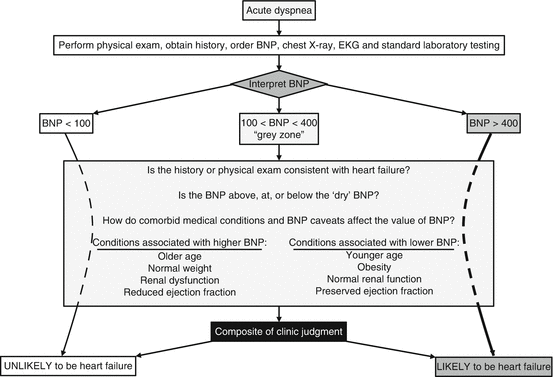

Fig. 12.4
Algorithm for the evaluation of acute dyspnea using BNP. BNP cannot be used in isolation to make the diagnosis of heart failure. Interpreting BNP in the context of comorbid medical conditions and knowledge of the caveats of BNP measurement is paramount to the accurate diagnosis of heart failure. BNP values are in pg/mL
12.7 Emerging Biomarkers in Heart Failure
In recent years, several new biomarkers have been studied as diagnostic and prognostic markers for heart failure. In the future, these additional biomarkers may be useful in the diagnosis of heart failure when used with BNP or NT-proBNP, particularly in patients that fall within the “gray zone.” In addition, biomarkers to help establish prognosis could be used for the triage of patients in the ED or in monitoring response to therapy.
12.7.1 Troponin
Troponin is well established as a marker of myocardial injury in acute myocardial infarction. Troponin T or troponin I is frequently ordered in patients admitted with acute heart failure. In a study of 84,872 patients admitted with decompensated heart failure from the ADHERE, a positive troponin was associated with lower systolic blood pressure, lower ejection fraction, and higher in-hospital mortality [49]. Although troponin is not useful in the diagnosis of heart failure, it has independent prognostic value in predicting in-hospital mortality in heart failure (Chaps. 10 and 11).
12.7.2 Copeptin
Natriuretic peptides are one of several neurohormonal systems activated by heart failure. Arginine vasopressin (AVP), also termed antidiuretic hormone, is released by the hypothalamus, promotes renal water conservation, and has vasoactive properties. AVP is difficult to measure in serum because it is unstable and rapidly cleared. Copeptin is the C-terminal portion of provasopressin. It is more stable and easier to measure in serum than AVP while still secreted in equal amounts to AVP [50].
Copeptin is elevated in multiple conditions, including sepsis and hemorrhagic shock [51]. Used with NT-proBNP, copeptin is a good predictor of adverse events after acute myocardial infarction [52]. Although copeptin has limited diagnostic utility, it may be useful in the future as a prognostic marker for heart failure. Additionally, copeptin increases with severity of heart failure symptoms by NYHA class and may be better than BNP and NT-proBNP in heart failure when used as a prognostic marker for adverse events including mortality [53, 54].
12.7.3 MR-proANP
Mid-region pro-atrial natriuretic peptide (MR-proANP) is a stable fragment of the ANP propeptide and mirrors the release of the biologically active unstable ANP. MR-proANP is released in response to similar conditions as BNP and NT-proBNP. In the Biomarkers in Acute Heart Failure (BACH) trial, MR-proANP was highly sensitive and specific for the diagnosis of heart failure and performed similar to both BNP and NT-proBNP [55]. In addition to diagnostic utility, MR-proANP may have prognostic value as the change in MR-proANP over time was shown to be a predictor of mortality [56]. In the future, MR-proANP may be used as an adjunctive diagnostic study for subgroups where diagnosis is difficult, such as patients with obesity, with renal failure, or with “gray zone” levels of BNP.
12.7.4 MR-proADM
Adrenomedullin (ADM) is a peptide hormone expressed by many tissues and organ systems. It has natriuretic, vasodilatory, and potent hypotensive effects. ADM is elevated in patients with chronic heart failure and increases with disease severity [57, 58]. However, its clinical utility has been limited due to biologic instability. Recently, immunoassays to detect the stable prohormone fragments of ADM have been developed. Mid-region pro-adrenomedullin (MR-proADM) demonstrated prognostic potential in the BACH trial. In 568 patients with acute heart failure, MR-proADM was superior to both BNP and NT-proBNP at predicting mortality within 14 days. In addition, when used with BNP and NT-proBNP, MR-proADM had additive incremental predictive value for 90-day mortality [56]. Although more research must be done, MR-proADM could be a useful alternative to NPs for prognosis and risk stratification.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


