Myocarditis and Dilated Cardiomyopathy
The concept of myocarditis was first introduced by Corvisart in 1812. The term is broadly defined by an inflammatory infiltration of the myocardium with associated necrosis and/or degeneration.1 The disease is also known as “myocarditis with cardiac dysfunction” when left ventricular systolic dysfunction is evident. Although there is a tremendously wide spectrum of clinical presentation, it is frequently associated with acute-onset profound cardiac dysfunction leading to rapidly progressive heart failure (HF) and arrhythmia development in an otherwise healthy young person. Myocarditis is also one of the major causes of sudden cardiac death in patients < 20 years old.2
Epidemiology and Classification
The exact incidence and prevalence of myocarditis is unclear because the majority of cases of myocarditis may be subclinical in presentation. Nevertheless, myocarditis usually affects younger individuals (median age of 42 years), with a slight predominance of men.3 The estimated incidence of myocarditis is 1 to 10 per 100,000 persons from military recruits and autopsy studies, and about 1% to 5% of patients with acute viral infections may have some involvement in the myocardium.4 The incidence of biopsy-proven myocarditis ranges from 9% to 11% in adults and up to 38% in children with acute-onset HF5. The presence of viral genome in biopsies of patients with idiopathic dilated cardiomyopathy (IDCM) is up to 65% of the individuals, suggesting that viral subclinical myocarditis could be more frequent than suspected and may be the cause of many of the IDCM cases considered “idiopathic.”6
The classification of myocarditis is confusing but is often defined according to the description of the disease course as “fulminant,” “acute,” or “chronic” (Table 19.1).7 With the availability of endomyocardial biopsy techniques in the 1970s, a technical (histologic) definition has been standardized by pathologists:
TABLE
19.1 Clinicopathological Classification of Myocarditis
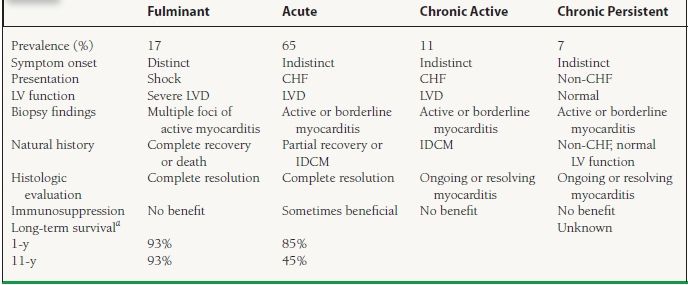
aLong-term survival data from 147 biopsy-proven myocarditis cases followed at the Johns Hopkins Hospital from 1984 to 1997.
IDCM, idiopathic dilated cardiomyopathy; LVD, LV dysfunction; LV, left ventricular.
Adapted from McCarthy RE III, Boehmer JP, Hruban RH, et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690-695, and Lieberman EB, Hutchins GM, Herskowitz A, et al. Clinicopathologic description of myocarditis. J Am Coll Cardiol. 1991;18:1617-1626.
1. The Dallas criteria (1987)8 describe the quantity and distribution patterns of lymphocyte infiltrates, and classify into three main types: (a) myocarditis (with or without fibrosis), (b) borderline myocarditis, and (c) no myocarditis. The availability of a second follow-up biopsy may allow further stratification into “ongoing myocarditis,” “resolving/healing myocarditis,” or “resolved myocarditis.”
2. The World Heart Foundation Marburg Criteria (1996)9 added a quantitative assessment of lymphocyte density (with the cutoff at 14 cells/mm2) for the stratification of the biopsies. Newer histologic criteria rely on immunohistologic quantification and characterization of immunocompetent infiltrates and cell adhesion molecule expression like anti-CD3, anti-CD4, anti-CD20, and anti-CD28. Criteria based on cellular staining have better sensitivity and could guide immunomodulatory therapy and also have a prognostic value.10,11
Etiologies and Pathophysiology
Many infectious and noninfectious agents can cause myocarditis (Table 19.2).4,12 Viral infection is the most common cause in North America and Europe, with enterovirus (including coxsackievirus) and adenovirus being classically the most frequently identified viruses.13,14 Recent data has raised the importance of parvovirus B19 (PVB19) and human herpesvirus-6 as causative agents, not only in biopsies from acute myocarditis patients15 but also when subjects with IDCM are studied. Pankuweit et al.16 described the persistence of viral genome of PVB19 in up to 33% of endomyocardial biopsy samples from patients with IDCM, ejection fraction (EF) < 45%, and persistent inflammation in their histology. A recent publication from Stewart et al. showed that PVB19 was the only virus isolated from tissue samples in adult HF patients referred for endomyocardial biopsy but in a lower rate than previously described (12%). Furthermore, they did not support a causative role for PVB19 persistence in the development of HF as there was a lack of correlation between PVB19 genome and HF progression in these patients.17 Otherwise, two or more viruses have also been described in more than 25% of patients with IDCM,6 suggesting a simultaneous synergic effect of the different viruses.
TABLE
19.2 Causes of Myocarditis

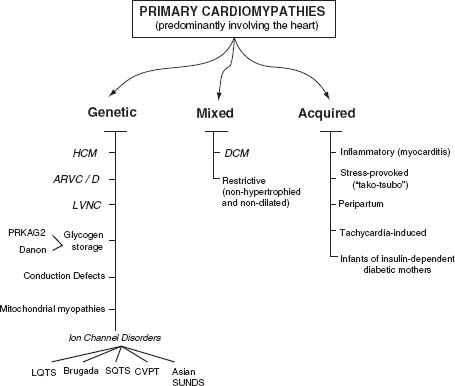
The precise pathogenic mechanisms of the disease are generally not well understood and may vary according to the causative agent and host factors. The majority of the cases are presumed to be due to a common pathway of host autoimmune-mediated injury. Direct cytotoxic effects of the causative agent plus damage due to myocardial cytokine expression and direct endothelium injury seem to be the key factors. Clinically, myocardial damage follows the expected course of inflammatory response (Fig. 19.1).4
FIGURE 19.1 Classification of primary cardiomyopathies predominantly involving the heart. (From Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807-1816, with permission.)
 Acute phase (0 to 3 days) is characterized by myocyte destruction and cardiac proteins exposure as a direct consequence of the offending agent. There is also cytokine expression and macrophage activation, leading to cell-mediated cytotoxicity and cytokine release that contribute to myocardial damage and dysfunction. In viral myocarditis, viremia is often present, although detection may sometimes be difficult.
Acute phase (0 to 3 days) is characterized by myocyte destruction and cardiac proteins exposure as a direct consequence of the offending agent. There is also cytokine expression and macrophage activation, leading to cell-mediated cytotoxicity and cytokine release that contribute to myocardial damage and dysfunction. In viral myocarditis, viremia is often present, although detection may sometimes be difficult.
 Subacute phase (4 to 14 days): Most patients recover after the acute phase but a subgroup progresses to this second stage that consists of an adaptive immune response. In addition to the continued cytokine production and myocyte destruction by nonspecific autoimmune-mediated injury (through cytotoxic T-and natural killer cells), antibodies are produced against viral proteins (in the subgroup patients with viral myocarditis) and cardiac proteins (including β1 receptor and cardiac myosin).
Subacute phase (4 to 14 days): Most patients recover after the acute phase but a subgroup progresses to this second stage that consists of an adaptive immune response. In addition to the continued cytokine production and myocyte destruction by nonspecific autoimmune-mediated injury (through cytotoxic T-and natural killer cells), antibodies are produced against viral proteins (in the subgroup patients with viral myocarditis) and cardiac proteins (including β1 receptor and cardiac myosin).
 Chronic phase (>14 days) involves a repair process characterized by fibrosis, and downregulation of the immune response. Some patients may have persistence of autoantibodies and also persistence of viral genome in myocardium. It has been estimated that between 20% and 50% of patients with acute myocarditis can progress to this chronic phase and develop cardiac dilatation and chronic HF.18 Those cases where inflammation persists on endomyocardial biopsy are considered as “chronic active myocarditis” or “persistent myocarditis.” A subclassification of the cardiomyopathy cases with persistent viral genome has been proposed. The patients in which viral clearing fails and have inflammatory disease in the biopsy would be referred as “viral inflammatory cardiomyopathy.” Those cases with dilated cardiomyopathy where inflammation is absent would correspond to a “viral cardiomyopathy.”19
Chronic phase (>14 days) involves a repair process characterized by fibrosis, and downregulation of the immune response. Some patients may have persistence of autoantibodies and also persistence of viral genome in myocardium. It has been estimated that between 20% and 50% of patients with acute myocarditis can progress to this chronic phase and develop cardiac dilatation and chronic HF.18 Those cases where inflammation persists on endomyocardial biopsy are considered as “chronic active myocarditis” or “persistent myocarditis.” A subclassification of the cardiomyopathy cases with persistent viral genome has been proposed. The patients in which viral clearing fails and have inflammatory disease in the biopsy would be referred as “viral inflammatory cardiomyopathy.” Those cases with dilated cardiomyopathy where inflammation is absent would correspond to a “viral cardiomyopathy.”19
In viral myocarditis, viral isolates differ in tissue tropism and virulence. For example, coxsackie A9 is a self-limiting myocarditis, whereas coxsackie B3 causes severe myocarditis with a high mortality. In addition, the induction of the coxsackie-adenovirus receptor (CAR) and the complement deflecting protein decay accelerating factor (DAF, CD55) may allow efficient internationization of the viral genome.20 These key molecular determinants for cardiotropic viral infections can be found in up to two-thirds of patients with IDCM. Viral replication may lead to further disruption of metabolism and perturbation of inflammation and its response. More recent evidence of dystrophin disruption by expression of enteroviral protease 2A points to yet another unique pathogenic mechanism.21 Also, endothelial cells have been recognized as a target for PVB19 infection if they express blood group P antigen, which serves as a cellular receptor for this virus. Therefore, initial parvovirus infection of endothelial cells of small coronary arteries may cause endothelial dysfunction, vasospasm, and ischemia. This may be a cofactor for myocardial damage progression and could also mimic myocardial infarction presentation, what has been called “parvomyocarditis.”22 Interestingly, other investigators have not found histopathologic signs of chronic ischemic disease like subendocardial fibrosis and vacuolization on endomyocardial biopsy of HF patients with a positive tissue PCR for PVB19. These findings contradict the theory of endothelial and ischemic damage related to PVB19.17
Clinical Presentation
Myocarditis can be totally asymptomatic or can present with a chest pain syndrome ranging from the mild persistent chest pain of acute myopericarditis (35%) to severe symptoms that resemble myocardial ischemia.4 About 60% of patients may have history of arthralgias, malaise, fevers, sweats, or chills consistent with viral infections (pharyngitis, tonsillitis, upper respiratory tract infection) usually about 1 to 2 weeks prior to onset. The hallmark symptoms of acute or fulminant myocarditis are those of acute-onset HF in a person without known cardiac dysfunction or with low cardiovascular risks. The diagnosis is usually presumptive, based on patient demographics and the clinical course (spontaneous recovery following supportive care or death). Patients with an acute HF presentation usually will have tachycardia with a S3 gallop, jugular venous distension, and peripheral edema. An audible pericardial friction rub may accompany in cases of myopericarditis. In some instances, patients may present with arrhythmia in the form of palpitations caused by supraventricular or ventricular tachyarrhythmia, syncope caused by heart block (“Stokes–Adams attack”) or sudden cardiac death.
Additional findings may accompany specific forms of myocarditis. In patients with acute rheumatic fever, associated signs include erythema marginatum, polyarthralgia, chorea, and subcutaneous nodules (Jones criteria for rheumatic fever). In cases of sarcoid myocarditis, lymphadenopathy and arrhythmias are common (up to 70% of affected individuals). Chagas acute infection may present with arrhythmias and cardiac conduction abnormalities. Hypersensitive or eosinophilic myocarditis is often associated with a pruritic maculopapular rash (and history of offending drug use) and eosinophilia in their blood work. The typical presentation of a patient with giant-cell myocarditis involves sustained ventricular tachycardia and rapidly progressive HF leading to cardiogenic shock. These features have low specificities but are often useful and may raise the suspicion of underlying myocarditis.
Evaluation
Inflammation is the hallmark feature of myocarditis. Clinically, an early onset of fever, tachycardia, hypotension, reduced ventricular function, elevated acute phase reactants (erythrocyte sedimentation rate or C-reactive protein), leukocytosis, and increased cardiac enzymes (CK-MB/cardiac troponins) are predictive of myocarditis. However, the prevalence of an increased troponin T in biopsy-proven myocarditis is only 35% to 45%.23 A lower level of troponin I at admission has been associated with an increased risk for death, heart transplantation, or persistent ventricular dysfunction in patients with fulminant myocarditis.24 The presence of eosinophilia may suggest hypersensitive (eosinophilic) myocarditis. Novel inflammatory markers that are still under investigation include tumor necrosis factor (TNF)-α, interleukin (IL)-10, serum-soluble Fas, and soluble Fas-ligand levels.25,26 Elevation of these markers portends a worse prognosis, although they are rarely used in the clinical setting. Serum viral antibody titers are usually increased fourfold or more in the acute phase and gradually fall during convalescence. However, measurement of viral antibody titers is infrequently indicated due to the usual low viral levels at the time of HF presentation and the lack of evidence for antiviral therapy. Because of their low specificity, measurement of anticardiac antibody titers is not indicated (only 62% of myocarditis cases have titers ≥1:40). Screening antinuclear antibodies and rheumatoid factor are often indicated to rule out common rheumatologic problems. Disease-specific testing is indicated if particular conditions such as systematic lupus erythematosus, polymyositis, Wegner granulomatosus, or scleroderma are suspected.
The electrocardiogram generally reveals sinus tachycardia, although sometimes ST-segment deviation can be found, making it necessary to rule out ischemia especially in patients with cardiovascular risk factors. In some cases, fascicular block, atrioventricular conduction disturbances, or ventricular tachyarrhythmias may be hemodynamically significant. A complete echocardiogram is a standard procedure for patients with suspected myocarditis to (a) exclude alternative causes of HF-like valvular disease, (b) quantify the degree of left ventricular dysfunction to monitor response to therapy, and (c) detect the presence of intracardiac thrombi. Occasionally, focal wall motion abnormalities and presence of pericardial fluid may prompt further workup or intervention. Fulminant myocarditis is often characterized by near normal diastolic dimensions and increased septal wall thickness, whereas acute myocarditis often has increased diastolic dimensions but normal septal wall thickness.27 Coronary angiography is often performed to rule out coronary disease as cause of new-onset HF in patients with risk factors or with a clinical presentation that mimic myocardial ischemia (“pseudoinfarct pattern”). This is especially relevant in the presence of focal wall motion abnormalities on echocardiography and localizing electrocardiographic changes. Several specialized imaging procedures are available to detect the presence of myocarditis, although they are rarely used clinically. Antimyosin scintigraphy using indium-III monoclonal antimyosin antibody provides identification of myocardial inflammation, with a high sensitivity (91% to 100%) and negative predictive value (93% to 100%) but relatively low specificity (28% to 33%) of detecting myocarditis. Gallium scanning has been utilized to identify severe myocardial cellular infiltration with high specificity (98%) but low sensitivity (36%).4
Gadolinium-enhanced cardiac magnetic resonance imaging (MRI) is being used with increasing frequency for noninvasive evaluation of patients with suspected myocarditis. Cardiac MRI can assess different markers of tissue injury, including intracellular and interstitial edema, capillary leakage with hyperemia, and cellular necrosis with fibrosis. The International Group on Cardiovascular Magnetic Resonance in Myocarditis recommended to perform a cardiac MRI when the patient was symptomatic, if the clinical suspicion of myocarditis was high, and if the MRI result will likely impact clinical management. The authors proposed three tissue markers (the “Lake Louise Criteria”) to confirm the diagnosis of myocarditis (Table 19.3). If all sequences can be performed and two or more of the three tissue-based criteria are positive, myocardial inflammation can be predicted with a diagnostic accuracy of 78%; if only late gadolinium enhancement imaging is performed, the diagnostic accuracy falls to 68%.28
TABLE
19.3 Cardiac MRI Diagnostic Criteria for Myocarditis
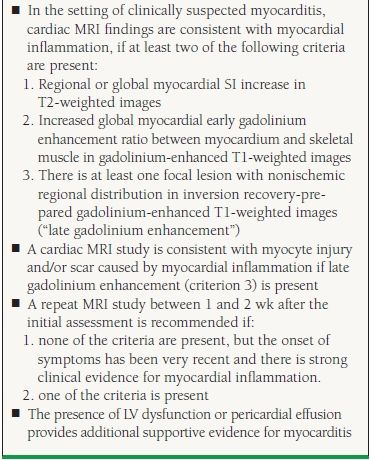
Reprinted from Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475-1487, with permission from Elsevier.