Failure of heart to pump blood forward at sufficient rate to meet metabolic demands of peripheral tissues, or ability to do so only at abnormally high cardiac filling pressures
Low output (↓ cardiac output) vs high output (↑ stroke volume ± ↑ cardiac output)
Left-sided (pulmonary edema) vs right-sided (↑ JVP, hepatomegaly, peripheral edema)
Backward (↑ filling pressures, congestion) vs forward (impaired systemic perfusion)
Systolic (inability to expel sufficient blood) vs diastolic (failure to relax and fill normally)
Some degree of systolic and diastolic dysfxn may occur regardless of ejection fraction
Congestion (“dry” vs “wet”)
⊕ hepatojugular reflux: ≥4 cm ↑ in JVP that persists for ≥15 sec w/ abdominal pressure Se/Sp 73/87% for RA >8 and Se/Sp 55/83% for PCWP >15 (AJC 1990;66:1002)
Abnl Valsalva response: square wave (↑ SBP w/ strain), no overshoot (no ↑ BP after strain) S3 (in Pts w/ HF → ˜40% ↑ risk of HF hosp. or pump failure death; NEJM 2001;345:574) rales, dullness at base 2° pleural effus. (often absent in chronic HF due to lymphatic compensation) ± hepatomegaly, ascites and jaundice, peripheral edema
Perfusion (“warm” vs “cold”): narrow pulse pressure (<25% of SBP) → CI <2.2 (91% Se, 83% Sp; JAMA 1989;261:884); other signs of low perfusion include soft S1 (↑ dP/dt), pulsus alternans, cool & pale extremities, ↓ UOP, muscle atrophy
CXR (see Radiology insert): pulm edema, pleural effusions ± cardiomegaly, cephalization, Kerley B-lines
BNP/NT-proBNP can help exclude HF; levels ↑ w/ age, renal dysfxn, AF, ↓ w/ obesity Se ≥95%, Sp ˜50%, PPV ˜65%, NPV ≥94% for HF in Pts p/w SOB (BMJ 2015;350:h910)
Echo (see inserts): ↓ EF & ↑ chamber size ↑ systolic dysfxn; hypertrophy, abnl MV inflow, abnl tissue Doppler → ? diastolic dysfxn; abnl valves or pericardium; ↑ estimated RVSP
ECG: Q waves or PRWP (ischemic heart disease); LVH (hypertensive heart disease or HCM); low limb lead voltage (NICM or infiltrative); heart block (infiltrative)
TTE: LV & RV size & function, valvular disease (and whether likely 1° or 2° to CMP), findings indicative of infiltrative or pericardial disease
Cardiac MRI: multiparameter evaluation of cardiac structure & function including:
LVEF, RVEF and volumes; regional wall motion abnormalities
presence, pattern, & extent of myocardial scar (using late gadolinium enhancement, LGE) w/ Se/Sp of 100%/96% vs coronary angio for dx etiology of HF (Circ 2011;124:1351)
myocardial inflammation or infiltration (eg, myocarditis, sarcoidosis, amyloidosis) restriction vs constrictive pericardial disease
| |||||||||||||||||||||
Dietary indiscretion or medical nonadherence (˜40% of cases)
Myocardial ischemia or infarction (˜10-15% of cases); myocarditis
Renal failure (acute, progression of CKD, or insufficient dialysis) → ↑ preload
Arrhythmias; acute valv. dysfxn (eg, endocarditis), espec mitral or aortic regurgitation
Other: extreme emotional stress; anemia, systemic infection, thyroid disease
For congestion: “LMNOP”
Lasix IV w/ monitoring of UOP; total daily dose 2.5× usual daily PO dose → ↑ UOP, but transient ↑ in renal dysfxn vs 1× usual dose; Ø clear diff between cont gtt vs q12h dosing (NEJM 2011;364:797)
Morphine (↓ sx, venodilator, ↓ afterload)
Nitrates (venodilator)
Oxygen ± noninvasive vent (↓ sx, ↑ PaO2; no Δ mortality; see “Mechanical Ventilation”)
Position (sitting up & legs dangling over side of bed → ↓ preload)
For low perfusion, see below
Adjustment of oral meds
| ||||||||||||||||||
Utility of BNP-guided Rx remains debated (Circ 2013;301:500 & 509)
IV vasodilators: NTG, nitroprusside (risk of coronary steal if CAD; prolonged use → cyanide/thiocyanate toxicity); nesiritide (rBNP) not rec for routine use (NEJM 2011;365:32)
Inotropes: in addition to ↑ inotropy, consider additional properties:
dopamine: splanchnic vasodil. → ↑ GFR & natriuresis; vasoconstrictor at ≥5 mcg/kg/min; does not enhance decongestion or preserve renal fxn in ADHF (JAMA 2013;310:2533)
milrinone: prominent systemic & pulmonary vasodilation; ↑ dose by 50% in renal failure
Ultrafiltration: similar wt loss to aggressive diuresis, but ↑ renal failure (NEJM 2012:367:2296)
Temporary MCS: depending on the device (Table), can be placed percutaneously or surgically to support LV or RV, as a bridge to recovery, for periprocedural support, or as a bridge to decision regarding transplant or durable long-term MCS
Intra-aortic balloon pump (IABP): inflates in diastole & deflates in systole to ↓ impedance to LV ejection of blood, ↓ myocardial O2 demand & ↑ coronary perfusion
Axial flow pumps (eg, Impella): Archimedes screw principle in LV
Extracorporeal magnetically levitated centrifugal pumps (eg, TandemHeart & CentraMag)
Extracorporeal membrane oxygenation (ECMO, Circ 2015;131:676)
Short-Term Mechanical Circulatory Support in Cardiogenic Shock
Device
Impella 2.5 & CP
Impella 5.0
Tandem
CentriMag
Max support (L/min)
0.5
2.5 & 3-4
5.0
5.0
10
6
RV support
N
Y (2nd dev.)
N
Y (2nd dev.)
Y (2nd dev.)
Y
Support duration
wks
˜4 wk
˜4 wk
<4 wk
mos
wks
Percutan?
Y
Y
N
Y
N
Y
Contraindic.
coagulop severe PAD
coagulop
coagulop
(Circ 2011;123:533 & 126:1717; JACC 2015;65:e7)
Durable Long-term MCS: surgically placed LVAD ± RVAD as bridge to recovery (NEJM 2006;355:1873) or transplant (HeartMate II or HeartWare LVAD or Total Artificial Heart if biventricular failure), or as destination Rx (HeartMate I: 52% ↓ 1-y mort. vs med Rx; NEJM 2001;345:1435, HeartMate II: 24% ↑ mort. vs HeartMate 1; NEJM 2009;361:2241)
Cardiac transplantation: curative Rx but supply of organs limited (˜2500/y in U.S.) 10% mortality in 1st year, median survival ˜10 y
Contraindications: active malignancy or infection, irreversible PHT (TPG>15, PVR>5WU despite pulmonary vasodilator Rx), active substance abuse or lack of social supports
Relative contraindications: age (eg, >70 y), other end organ dysfunction (liver, kidney, lung, unless dual organ transplantation is performed)
| ||||||||||||||||||||||||||||||||||||||||
Epidemiology: ˜½ of Pts w/ HF have normal or only min. impaired systolic fxn (EF ≥40%); risk factors for HFpEF incl ↑ age, ♀, DM, AF. Mortality ≈ to those w/ systolic dysfxn.
Etiologies (impaired relaxation and/or ↑ passive stiffness): ischemia, prior MI, LVH, HCMP infiltrative CMP, RCMP aging, hypothyroidism
Precipitants of pulmonary edema: volume overload (poor compliance of LV → sensitive to even modest ↑ in volume); ischemia (↓ relaxation); tachycardia (↓ filling time in diastole), AF (loss of atrial boost to LV filling); HTN (↓ afterload → ↓ stroke volume)
(1) echo: abnl MV inflow (E/A reversal and Δs in E wave deceleration time) & ↓ myocardial relax. (↑ isovol relax. time & ↓ early diastole tissue Doppler velocities)
(2) exercise-induced ↑ PCWP (± ↓ response chronotropic & vasodilator reserve)
Treatment: diuresis for vol overload, BP control, prevention of tachycardia and ischemia; no benefit to: ACEI/ARB (NEJM 2008;359:2456) or PDE5 inhib (JAMA 2013;309:1268) spironolactone ↓ CV death & HF hosp (at least in Americas) (NEJM 2014;370:1383);
Cardiac output (CO) = SV × HR; LV SV depends on LV end-diastolic volume (LVEDV) manipulate LVEDV to optimize CO while minimizing pulmonary edema
manipulate LVEDV to optimize CO while minimizing pulmonary edema
Balloon at tip of catheter inflated → floats into “wedge” position. Column of blood extends from tip of catheter, through pulmonary circulation, to a point just proximal to LA. Under conditions of no flow, PCWP ≈ LA pressure ≈ LVEDP, which is proportional to LVEDV.
Situations in which these basic assumptions fail:
(1) Catheter tip not in West lung zone 3 (and PCWP = alveolar pressure ≠ LA pressure); clues include lack of a & v waves and if PA diastolic pressure < PCWP
PCWP = alveolar pressure ≠ LA pressure); clues include lack of a & v waves and if PA diastolic pressure < PCWP
Diagnosis and evaluation
Ddx of shock (cardiogenic vs distributive; espec if trial of IVF failed or is high risk) and of pulmonary edema (cardiogenic vs not; espec if trial of diuretic failed or is high risk)
Therapeutics (Circ 2006;113:1020)
Guide to perioperative management in some high-risk Pts, pretransplantation
Guide to candidacy for thoracic organ transplantation and mechanical circulatory support
Contraindications
Absolute: right-sided endocarditis, thrombus/mass or mechanical valve; PE
No benefit in decompensated HF (JAMA 2005;294:1625); untested in cardiogenic shock
| |||||||||||||||||||||||||||||||||
Central venous access: pneumo/hemothorax (˜1%), arterial puncture (if inadvertent cannulation w/ dilation → surgical/endovasc eval), air embolism, thoracic duct injury
Inability to advance: difficulty RA → RV seen w/ lg RA and/or severe TR; difficulty RV → PA seen w/ lg RV; deflate balloon, withdraw 10 cm, inflate balloon & readvance. If repeated attempts unsuccessful at bedside, then perform under fluoro (to avoid PAC coiling & potentially knotting, which would require interventional cardiology to extract).
PA rupture: risk factors include prolonged balloon inflation, PHT, anticoag. Presents w/ hemoptysis and, if severe, hypoxemia & hypovolemic shock. Keep balloon inflated, intubate w/ dbl-lumen endotracheal tube, lateral decubitus position with affected side down, STAT interventional radiology and/or thoracic surgery consultation.
Trendelenburg, high-flow O2.
Zero the transducer and level it with the right atrium (phlebostatic axis)
Transmural pressure (≈ preload) = measured intracardiac pressure – intrathoracic pressure
Intrathoracic pressure (usually slightly [circled dash]) is transmitted to vessels and heart
Always measure intracardiac pressure at end-expiration, when intrathoracic pressure closest to 0 (“high point” in spont. breathing Pts; “low point” in Pts on ⊕ pressure vent.)
If ↑ intrathoracic pressure (eg, PEEP), measured PCWP overestimates true transmural pressures. Can approx by subtracting ˜½ PEEP (× ¾ to convert cm H2O to mmHg).
Thermodilution: saline injected in RA. Δ in temp over time measured at thermistor (in PA) is integrated and is ≈ 1/CO. Inaccurate if ↓ CO, severe TR or shunt.
[V with dot above]O2 ideally measured (espec if ↑ metab demands), but freq estimated (125 mL/min/m2)
C(a-v)O2 = [10 × 1.36 mL O2/g of Hb × Hb g/dL × (SaO2-SMVO2)]
| |||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
Goals: optimize both MAP and CO to promote end-organ perfusion, while ↓ risk of pulmonary edema & systemic venous congestion MAP = CO × SVR; CO = HR × SV (depends on preload, afterload and contractility) pulmonary edema when PCWP >20-25 (↑ levels may be tolerated in chronic HF) hepatic and renal congestion when CVP/RAP >15 mmHg
Optimize preload = LVEDV ≈ LVEDP ≈ LAP ≈ PCWP (NEJM 1973;289:1263) goal PCWP ˜14-18 in acute MI, ≤14 in acute decompensated HF optimize in individual Pt by measuring SV w/ different PCWP to create Starling curve ↑ by giving NS (albumin w/o clinical benefit over NS; PRBC if significant anemia) ↓ by diuresis (qv), ultrafiltration or dialysis if refractory to diuretics
Optimize afterload ≈ wall stress during LV ejection = [(˜SBP × radius) / (2 × wall thick.)] and ∝ MAP and ∝ SVR = (MAP – CVP / CO); goals: MAP >60, SVR 800-1200 MAP >60 & SVR ↑ : vasodilators (eg, nitroprusside, NTG, ACEI, hydral.) or wean pressors MAP <60 & SVR ↑ (&
∝ MAP and ∝ SVR = (MAP – CVP / CO); goals: MAP >60, SVR 800-1200 MAP >60 & SVR ↑ : vasodilators (eg, nitroprusside, NTG, ACEI, hydral.) or wean pressors MAP <60 & SVR ↑ (&  CO ↓ ): temporize w/ pressors until can ↑ CO (see below) MAP <60 & SVR low/nl (&
CO ↓ ): temporize w/ pressors until can ↑ CO (see below) MAP <60 & SVR low/nl (& 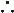 inappropriate vasoplegia): vasopressors (eg, norepinephrine [α, β], dopamine [D, α, β], phenylephrine [α] or vasopressin [V1] if refractory)
inappropriate vasoplegia): vasopressors (eg, norepinephrine [α, β], dopamine [D, α, β], phenylephrine [α] or vasopressin [V1] if refractory)
Optimize contractility ∝ CO for given preload & afterload; goal CI = (CO / BSA) >2.2 if too low despite optimal preload & vasodilators (as MAP permits):
⊕ inotropes: eg, dobutamine (mod inotrope & mild vasodilator) or milrinone (strong inotrope & vasodilator, incl pulm), both proarrhythmic, or epi (strong inotrope & pressor)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


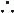 wait ≥40
wait ≥40 


 attractive if
attractive if  attractive if
attractive if 