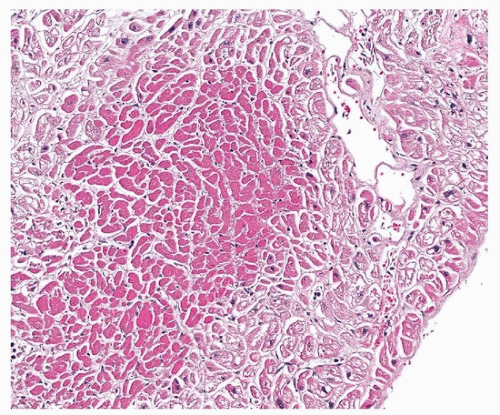
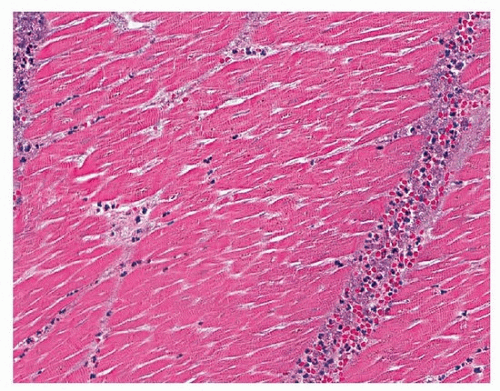
TABLE 139.1 Terms Used in Describing Myocardial Injury | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
mitochondria and that is inactivated by increased oxidative stress and apoptotic proteases, allowing for the programmed cell death to occur.
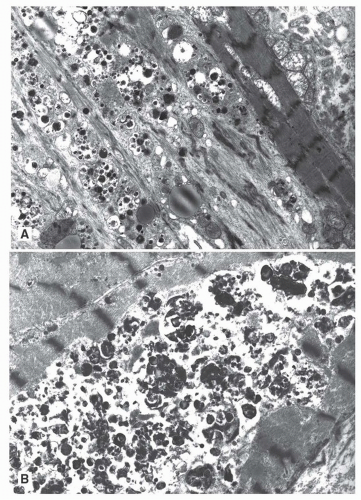 FIGURE 139.2 ▲ Autophagic vacuoles. A. Multiple adjacent cells are affected with myofibrillar loss and autophagic vacuoles. B. A portion of a myocyte is replaced by autophagic debris. |
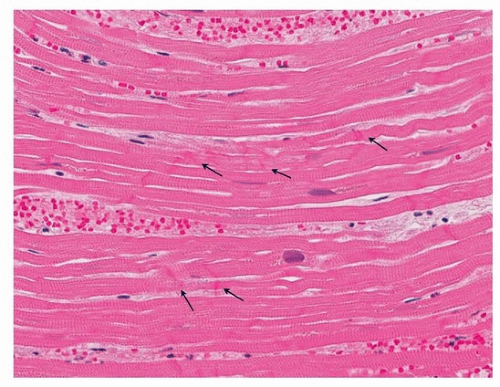 FIGURE 139.4 ▲ Contraction bands. There are thickened transverse bands corresponding to overlapping myofibrils (arrows). There is no evidence of necrosis.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|