13 Myocardial Pathology
Hypertrophic Cardiomyopathy
Background
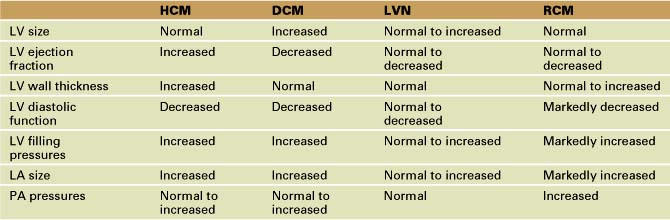
• Hypertrophic cardiomyopathy (HCM) is characterized by pathologic thickening of the left ventricle (LV) due to sarcomeric gene mutations.
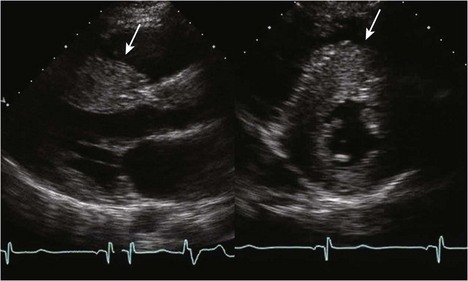
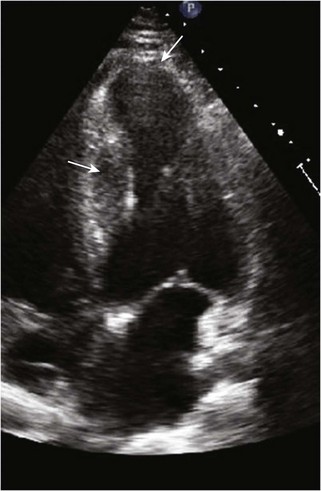
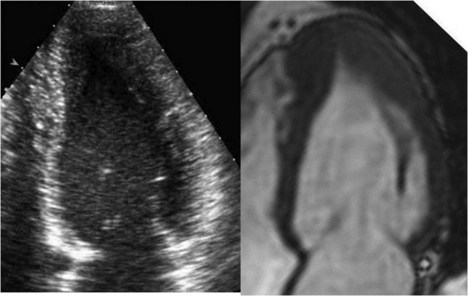
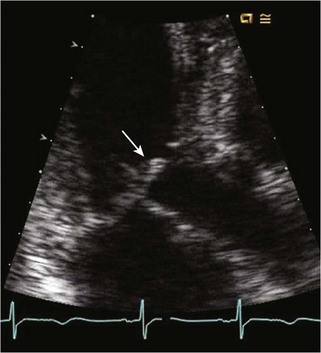
• HCM commonly involves asymmetrical hypertrophy of the anterior septum, but can manifest as concentric hypertrophy or hypertrophy of other segments (Figs. 13-1 through 13-3).
• Resting left ventricular outflow tract obstruction (LVOTO) due to systolic anterior motion of the mitral apparatus is present in about one third of HCM patients; another one third has provocable obstruction.
• A diagnosis of HCM is suggested by wall thickness greater than 1.5 cm in adults or a Z score indexed to body surface area of more than 2 in children (in the absence of other causes for hypertrophy).
• Structural or valvular abnormalities that increase left ventricular afterload (e.g., aortic stenosis, coarctation of the aorta) need to be excluded before a diagnosis of HCM can be made.
• Other conditions that mimic HCM include hypertensive heart disease, infiltrative disorders (e.g., amyloidosis), and glycogen storage disorders (e.g., Fabry disease, Danon disease, PRKAG2 mutations).
• In infants, 30% to 50% of cases of an HCM-like phenotype can be attributed to metabolic or syndromal disorders (e.g., Noonan syndrome). Infants born to diabetic mothers can have transient biventricular hypertrophy.
• Physiologic adaptation to exercise can lead to mild to moderate ventricular hypertrophy, which can mimic HCM. Features distinguishing “athlete’s heart” from HCM are shown in Table 13-1.
• Massive left ventricular hypertrophy (LVH)—wall thickness greater than 30 mm in adults or a Z score for body surface area greater than +15 in children—is a risk factor for sudden death in HCM patients.
TABLE 13-1 FEATURES DISTINGUISHING “ATHLETE’S HEART” FROM HCM IN ADULTS*
| Feature | Athlete’s Heart | HCM |
|---|---|---|
| Maximal wall thickness | ≤16 mm | ≥13 mm |
| Pattern of LVH | Predominantly concentric | Concentric or asymmetrical |
| LV cavity dimension | Often > 55 mm (in endurance athletes) | Usually < 45 mm |
| Diastolic function | Normal | Normal or abnormal |
| Gender | Male > female | Male = female |
| Family history of HCM or SCD | No | Yes or no |
| Delayed enhancement (MRI) | No | Yes or no |
| Exercise capacity | Above normal | Normal to below normal |
| Response to deconditioning | LVH regression | No change in LVH |
* Intended for adults or adult-sized teenagers. Corresponding Z scores can be calculated for children but have not been validated.
Echocardiographic Approach (Table 13-2)
Anatomic Imaging
• Step 1: Assess the magnitude and extent of LVH.
• Whenever possible, wall thickness measurements should be confirmed in more than one imaging plane (see Fig. 13-1).
• Short axis view imaging of the LV at multiple levels usually provides the best means of assessing wall thickness and overall hypertrophy.
• The right ventricular moderator band and papillary muscle insertions should not be included in wall thickness measurements. The parasternal long axis view is most susceptible to mismeasurement.
• Apical windows may be more suited to estimating LV wall thickness of the inferior septum and lateral wall, common regions of dropout on short axis view imaging.
• Step 2: Quantify systolic function.
• HCM patients often have an increase in circumferential shortening, but a decrease in longitudinal shortening.
• Hyperdynamic systolic function is also commonly seen in hypertensive heart disease or situations of decreased afterload.
• Step 3: Assess mitral leaflet motion for possible systolic anterior motion (SAM).
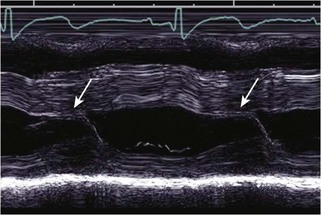
• M-mode depiction of mitral leaflet motion is essential for determining the timing of SAM and the duration of mitral-septal contract (Fig. 13-5).
• The presence of LVOTO in the absence of SAM suggests fixed subaortic stenosis, and additional causes of obstruction should be sought.
Physiologic Data
• Step 1: Assess the presence and severity of intracardiac obstruction.
• Obstruction can occur at either the midventricular or left ventricular outflow tract (LVOT) level.
• Midventricular obstruction predisposes to apical aneurysm formation and scarring; contrast agents should be considered if the apex is not well visualized.
• Changes in left ventricular geometry can alter intracardiac flow patterns, dragging the mitral leaflets anteriorly into the LVOT.
• Anterior movement of the tips of the mitral leaflets may result in a loss of coaptation and posteriorly directed mitral regurgitation (MR).
• Step 2: Measure the velocity of blood flow down the length of the LV using pulsed wave (PW) Doppler.
• Anterior displacement of the mitral leaflets causes associated MR to originate more anteriorly, and thus care must be taken to differentiate LVOT flow from regurgitation flow.
• Resting LVOT velocities greater than 5 m/s are uncommon and should be confirmed by finding a separate, even higher velocity (>7 m/s) mitral regurgitant jet.
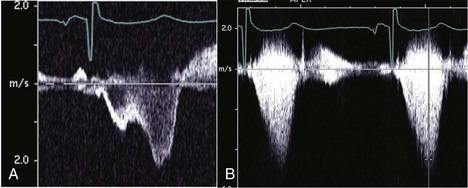
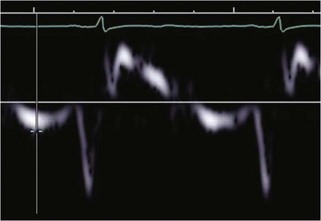
• Dynamic obstruction may produce a characteristic dagger-shaped, late-peaking Doppler waveform (Fig. 13-6A); however, complete LVOTO may produce a characteristic “lobster claw” appearance (Fig. 13-6B).
• Step 3: Assess changes in LVOT velocity with provocation in adults and older children.
• Provocation can unmask resting LVOT gradients; patients with provocable LVOTO are more likely to have exercise-induced obstruction.
• Step 4: Assess MR.
• MR can be difficult to quantify because of its timing (originating in mid to late systole) and eccentricity, making proximal isovelocity surface area (PISA) calculations less reliable.
• Step 5: Assess diastolic function.
• Assessment of diastolic function in HCM patients is challenging due to asymmetrical hypertrophy and changes in calcium kinetics.
• Because of the asymmetrical hypertrophy, mitral annular relaxation velocities should be sampled from the septal, lateral, anterior, and inferior aspects of the annulus, with an average velocity used to assess global diastolic function.
• The ratio of mitral inflow velocity deceleration (E wave) to early mitral annular relaxation (E′) velocity is not as robust a predictor of filling pressure as in other conditions.
Alternate Approaches
• Exercise echo is particularly useful in evaluating patients with suspected exercise-induced LVOTO.
• Cardiac magnetic resonance imaging (MRI) is useful for determining maximal wall thickness, and the use of delayed gadolinium enhancement can provide assessment of the extent of fibrosis.
• Consider the use of contrast agents for better visualization of the left ventricular apex in patients with midcavitary obstruction.
• 3D TEE can aid in the assessment of subvalvular architecture and the location of intracardiac obstruction.
Dilated Cardiomyopathy
• Dilated cardiomyopathy (DCM) is characterized by spherical enlargement of the LV with reduction in global systolic function. Four-chamber enlargement and dysfunction are often present (Fig. 13-8).
• DCM is often classified as ischemic (due to coronary artery disease) or nonischemic, based on the presence or absence of myocardial ischemia or infarction. Potential causes of DCM are listed in Table 13-3.
• Most cases of primary DCM in children are idiopathic, but a positive family history is present in 30% of cases, suggesting a genetic component.
• Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) can present with DCM in the first weeks of life due to decreasing PAP and resultant myocardial ischemia.
• Chamber enlargement can lead to annular dilation and leaflet tethering of the semilunar valves, causing secondary (functional) valve regurgitation.
• In contrast, a primary valvular cardiomyopathy (CM) should be suspected when significant aortic regurgitation (AR) is present in the setting of DCM.
• Diastolic dysfunction is commonly present in patients with impaired systolic function, with increase in LV filling pressures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree