Myocardial Infarction
Allen P. Burke, M.D.
Fabio R. Tavora, M.D., Ph.D.
Types of Acute Infarcts
Acute myocardial infarction indicates irreversible myocardial injury resulting in coagulative necrosis of the myocardium. Ischemia resulting in clinical or electrocardiographic manifestations is generally >1 cm.
“Acute” indicates infarction <5 days old, when the inflammatory infiltrate is primarily neutrophilic. Acute myocardial infarction may be either of the nonreperfusion type, in which case the obstruction to blood flow is permanent, or of the reperfusion type, in which the obstruction or lack of blood flow is long enough in duration (generally hours) but reversed or restored after there is myocardial cell death. “Subacute” of “healing” generally implies the onset of healing, as in granulation tissue with ingrowth of capillaries and fibrosis that begins at about 1 week. A “healed” infarct denotes the presence of dense scar, which begins at around 1 month, and is generally complete at 3 months.
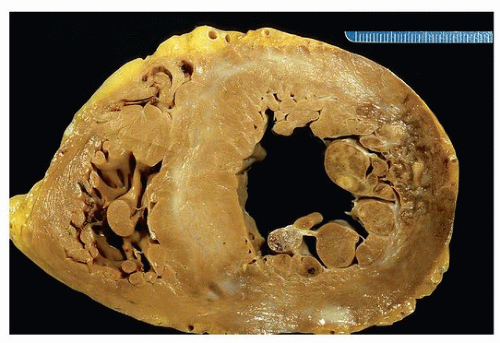
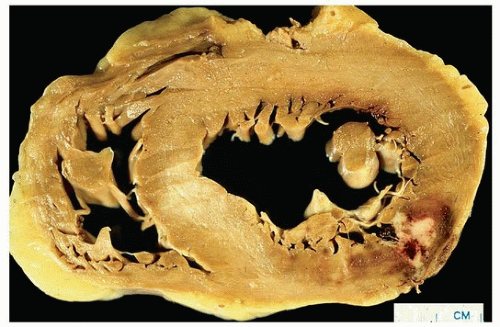
Grossly, infarcts are classified by extent into subendocardial infarcts (less than one-third to one-half of the myocardial thickness) and transmural infarcts. Ischemic lesions caused by epicardial occlusions are endocardial based.
Clinically, acute coronary syndromes are classified as unstable angina/non-ST elevation myocardial infarction (NSTEMI) and ST elevation myocardial infarction (STEMI). Troponin “leak” in the proper clinical setting is indicative of myocardial damage and is the basis for the distinction between unstable angina and NSTEMI.
Incidence
Acute myocardial infarctions are common and result in hospitalizations of approximately four men and two women per 1,000 people in the United States yearly.1
It has been estimated that an acute myocardial infarction occurs every 44 seconds in the United States.2 There is an annual incidence of 620,000 acute coronary events resulting in hospitalization or death and there are more than twice that rate of silent myocardial infarcts.2 Although the incidence has held steady in the United States, there has been a trend to a decrease in hospital mortality.1,2
Sudden Death and Heart Failure After Acute Infarction
Of all patients with STEMI, 25% to 35% will die of sudden cardiac death before receiving medical attention, most often from ventricular fibrillation. 3 Ventricular fibrillation complicates 5% to 6% of acute myocardial infarctions and imparts an increased risk of in-hospital mortality, but no increased long-term risk of sudden death.4
After hospital discharge for patients surviving acute myocardial infarction, there is a 1% to 2% risk of sudden death yearly for up to 5 years.5,6 The rate of sudden death is increased in patients with heart failure. For this reason, patients are often treated with implantation of a cardioverter defibrillator when malignant ventricular arrhythmias occur more than 48 hours after the infarction and when the ejection fraction is decreased.3
Up to one-third of patients have recurrent ischemia in a 5-year period, with half that rate developing heart failure. Heart failure is the most significant risk factor for sudden death.5
Etiology
Autopsies of hospital inpatients dying with acute myocardial infarction will reveal an acute thrombus overlying atherosclerotic plaque in over 95% of cases, with careful inspection of the coronary arteries.7,8 Most fatal infarcts are transmural STEMIs that are presumed caused by occlusive thrombi.9
In autopsy studies of sudden deaths, there is a range of data regarding the frequency of occlusive versus nonocclusive lesions. Occlusive lesions have been described at a rate of 30% to 40%.10,11 The thrombi in NSTEMIs have often been presumed to be primarily nonocclusive, although because they rarely result in deaths, confirmatory autopsy studies are lacking. More recent studies using intravascular ultrasound have shown a significant increase in plaque volume and thrombus size comparing unstable angina and acute myocardial infarction, but no difference between NSTEMI and STEMI other than the size of the arc of the ruptured plaque.12
In the remaining hearts in patients with acute myocardial infarction, which represents <5%, there will be severe coronary diseases without thrombus.13 Rare causes of acute myocardial infarction include no apparent cause (usually attributed to coronary spasm), coronary embolism (various causes, including valve vegetations and tumors), and thrombosis in nonatherosclerotic normal coronary arteries (hypercoagulable states) (see Chapter 155).
Reperfusion Infarction
Most myocardial infarcts in the preintervention era were nonreperfused, which indicates that the occlusion of a coronary artery is fixed, and not lysed by natural or iatrogenic means. Typically, a nonreperfused infarct is caused by an acute occlusive thrombus, which eventually organizes into a recanalized thrombus that may eventually allow some blood flow to occur.
When infarcts are diagnosed within hours of onset of symptoms, the thrombus is lysed pharmacologically or relieved by percutaneous intervention, preferably before irreversible injury occurs. If the infarct has resulted in irreversible injury prior to intervention (generally more than 6 to 8 hours after onset of chest pain), a reperfusion-type infarct occurs. In cases of reperfusion, the area at risk, or myocardium supplied by the thrombosed artery, is prone to irreversible ischemia from the occlusion itself as well as reperfusion injury. Components of reperfusion-induced myocardial damage include oxidative stress, Ca++ overload, rapid restoration of physiologic pH, and release of neutrophil granules, which open mitochondrial membranes and induce cardiomyocyte death.14 Histologically, revascularization of the necrotic myocardium results in hemorrhage and edema, and endothelial swelling brought by the diffuse influx of neutrophils.8
Distribution of Ischemia
Whether an infarct is of the nonreperfused or perfused type, a proximal occlusion at the level of an epicardial artery results in a typical distribution starting at the subendocardium and progressing toward the epicardium (so-called wavefront phenomenon).15 Therefore, an area of necrosis or scarring is considered to have an “ischemic pattern” if it is largest at the endocardium, with a wedge-shaped extending up to the epicardial surface. Ischemic injury may, however, be located in the midmyocardium or even subepicardium, if the level of the coronary occlusion is distal within the myocardium.8
The area of infarct occurs in the distribution of the occluded vessel (Table 151.1). Left main occlusion generally results in a large anterolateral infarct, whereas occlusion of the left anterior descending causes necrosis limited to the anterior wall. There is often extension to the anterior portion of the ventricular septum with proximal left coronary occlusions. In hearts with a right coronary dominance (right supplying
the posterior descending branch), a right coronary occlusion causes a posterior (inferior) infarct. With a left coronary dominance (about 15% of the population), a proximal circumflex occlusion will infarct the posterior wall; in the right dominant pattern, a proximal obtuse marginal thrombus will cause a lateral wall infarct only, and the circumflex distal is a small vessel. The anatomic variation due to microscopic collateral circulation, which is not evident at autopsy, plays a large factor in the size of necrosis and distribution. Unusual patterns of supply to the posterior wall, such as wraparound left anterior descending or posterior descending supplied by the obtuse marginal, may also result in unexpected areas of infarct in relation to the occluded proximal segment.
the posterior descending branch), a right coronary occlusion causes a posterior (inferior) infarct. With a left coronary dominance (about 15% of the population), a proximal circumflex occlusion will infarct the posterior wall; in the right dominant pattern, a proximal obtuse marginal thrombus will cause a lateral wall infarct only, and the circumflex distal is a small vessel. The anatomic variation due to microscopic collateral circulation, which is not evident at autopsy, plays a large factor in the size of necrosis and distribution. Unusual patterns of supply to the posterior wall, such as wraparound left anterior descending or posterior descending supplied by the obtuse marginal, may also result in unexpected areas of infarct in relation to the occluded proximal segment.
TABLE 151.1 Expected Distribution of Infarct, by Occluded Epicardial Vessel | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Gross Pathologic Findings
The earliest change that can be grossly discerned in the evolution of acute myocardial infarction is pallor of the myocardium, which is evident 12 hours or later after the onset of irreversible ischemia. The gross detection of infarction can be enhanced by the use of tetrazolium salt solutions, which form a colored precipitate on gross section of fresh heart tissue in the presence of dehydrogenase-mediated activity. Myocardial necrosis can be detected as early as 2 to 3 hours by this method.
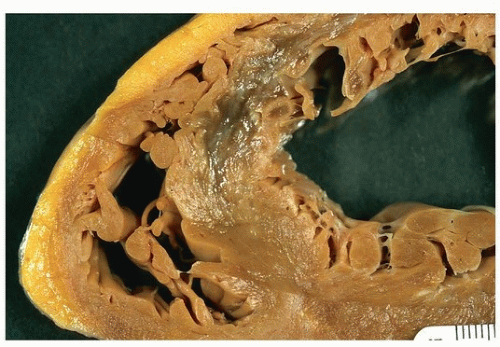
In nonreperfused infarction, the area of the infarct is well defined at 2 to 3 days with a central area of yellow discoloration that is surrounded by a thin rim of highly vascularized hyperemia. In a reperfused infarct, the infarcted region will appear red from trapping of the red cells and hemorrhage from the rupture of the necrotic capillaries.
At 5 to 7 days, the regions are much more distinct, with a central soft area and a depressed hyperemic border. At 1 to 2 weeks, the infarct begins to be depressed, especially at the margins where organization takes place, and the borders are white; a clear gray color may be seen at the granulation tissue border. Healing may be complete as early as 4 to 6 weeks in small infarcts, or may take as long as 2 to 3 months when the area of infarction is large.
Healed infarcts are white from the scarring, and the ventricular wall may be thinned (aneurysmal), especially in transmural infarction.
The size of the infarct, whether acute, healing, or healed, should be estimated my assessing the largest dimension in any slice and giving an extent of the infarct from apex to the base of the heart. For example, an infarct could be described as 4 cm in greatest dimension, extending from 1 cm from the apex to midmyocardium, involving three contiguous 1-cm slides.
Figures 151.1, 151.2, 151.3 show examples of gross findings in different stages of acute myocardial infarction.
Histopathology of Nonreperfused Infarcts
The border zone between necrotic myocardium and viable myocardium is the focus of dating, which depends on the reaction of viable myocardium to the area of infarct (Table 151.2).
TABLE 151.2 Histologic Evolution of Nonreperfused Myocardial Infarctiona
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|