(1)
University of Ottawa The Ottawa Hospital, Ottawa, ON, Canada
Overview
Acute coronary syndrome [ACS = ST-segment elevation myocardial infarction (STEMI) or non-ST-elevation myocardial infarction (NSTEMI) and unstable angina] is a life-threatening condition (see Fig. 11-1).
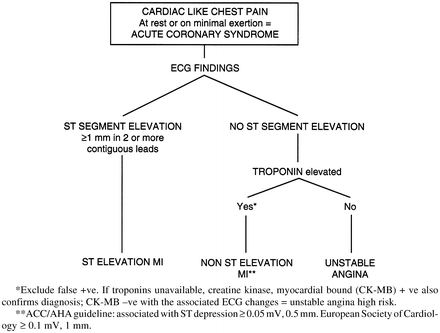
Fig. 11-1.
Diagnosis of ST-segment elevation MI (STEMI) and non-ST-segment elevation MI (NSTEMI) Acute coronary syndrome. *Exclude false +ve. If troponins are unavailable, creatine kinase, myocardial bound (CK-MB) +ve also confirms diagnosis; CK-MB –ve with the associated ECG changes = unstable angina high risk. **ACC/AHA guideline: associated with ST depression ≥0.05 mV, 0.5 mm in two or more contiguous leads. European Society of Cardiology ≥0.1 mV, 1 mm.
Patients presenting with ACS are at high risk for cardiac events: death which may be sudden, recurrence of Myocardial Infarction (MI), or ischemia.
Events can be significantly reduced by percutaneous coronary intervention [PCI] or thrombolytic therapy accomplished within 2–3 h of onset of MI. Ways to reduce delays in doing percutaneous coronary intervention soon after STEMI onset include early recognition of symptoms by patients and prehospital diagnosis by paramedics so that the emergency room can be bypassed in favor of direct admission to the catheterization laboratory (Windecker et al. 2013). The late Dr JF Pantridge, founder of coronary care units (1967), uttered the statement: “He advocated that a cardiac defibrillator should be considered in the same light as a fire extinguisher and thus should be available in most homes, place of work and sports arenas” (Pantridge 1974, personal conversation).
An acute MI remains a fatal event in >33 % of patients. Approximately 50 % of deaths occur within 1 h of onset of symptoms, mainly because of ventricular fibrillation (VF).
Of 55 million deaths globally every year, about 30 % are from cardiovascular diseases. Of these, 40–50 % are likely to be due to acute MI (Yusuf et al. 2001).
About 15 % of patients will either have died or had another MI or significant ischemia within 6 months following acute STEMI or NSTEMI.
The incidence of AMI is similar in Europe; unfortunately, the incidence is increasing in Asia and Latin America.
Although more than 50 million American adults have some atheromatous coronary artery disease (CAD), only a small fraction will ever develop erosion, fissuring, or plaque rupture that culminates in AMI (Topol 2005)
LIFESTYLE: Willett (2002) estimated that more than 80 % of coronary artery disease (CAD) may be accountable for by lifestyle issues: weight, diet, exercise, and control of risk factors such as blood pressure and smoking. There is evidence that a small consumption of alcohol, 1 drink for women and 2 for men, confers some cardiovascular protection. But a Prospective Study and Dose-Response Meta-Analysis indicated that alcohol consumption, even at moderate intakes, is a risk factor for atrial fibrillation. All studies reported a positive association, with an overall 8 % (6–10 %) increase in AF risk per 1 drink/day increment in alcohol consumption. In this large prospective study, both moderate (1–3 drinks/day) and high (>3 drinks/day) alcohol consumption was associated with increased AF risk. With regard to specific alcoholic beverages, consumption of liquor and wine but not beer was significantly positively associated with AF risk (Larsson et al. 2014).
FAMILY HISTORY: Nevertheless the evidence for heritability of AMI is striking, with a positive family history being one of the most important risk factors for this complex trait (Wang et al. 2004). Genetic studies indicate that the heritability of AMI is much more impressive than that of atherosclerotic CAD (Willett 2002; Topol et al. 2001), which in the majority remains stable and without plaque erosion or rupture.
TRIGGERS for ACS
Cocaine is the most likely to trigger an event in an individual, although only 0.9 % of MI cases were estimated to be triggered by cocaine.
Traffic, has the greatest population effect as more people are exposed to the trigger (Nawrot et al. 2011). Most city dwellers are exposed to traffic pollution which is now believed to be a major trigger for heart attacks and supersedes other triggers such as physical exertion in relatively sedentary individuals, air pollution, and anger.
Importantly, drivers of vehicles in heavy city traffic are exposed to exhaust fumes which circulate within their vehicles. It is not surprising therefore that in China, India, and other countries there is a rise in the occurrence of ACS as large population groups move from country living and become city dwellers.
LDL-C: The importance of maintaining LDL-C at goal levels in patients at intermediate and high risk cannot be overemphasized. Low HDL-C is a novel lipid phenotype that appears to be more prevalent among Asian populations, in whom it is associated with increased coronary risk (Huxley et al. 2011). Some genetic mechanisms that raise plasma HDL cholesterol, however, do not lower risk of MI. These data challenge the concept that raising of plasma HDL cholesterol will uniformly translate into reductions in risk of MI (Voight et al. 2012).
Serum Potassium stability is most important. Among inpatients with AMI, the lowest mortality was observed in those with post-admission serum potassium levels 3.5–4.4 mEq/L compared with those who had higher or lower potassium levels. Rates of ventricular fibrillation (VF) or cardiac arrest were higher only among patients with potassium levels of less than 3.0 mEq/L and at levels of 5.0 mEq/L or greater (Goyal et al. 2012).
DIAGNOSIS
Patients with symptoms of STEMI (chest discomfort with or without radiation to the arms, neck, jaw, epigastrium, or back; shortness of breath; weakness; diaphoresis; nausea; light-headedness) should be transported to the hospital by ambulance rather than by relatives. If nitroglycerin is available, one tablet or two puffs sublingual should be used and three chewable aspirins (total 240 mg) are taken while awaiting the ambulance.
Thuresson et al. (2005) point out that the typical symptom onset of AMI is observed in less than 50 % of patients with STEMI; only one in five fulfill all the criteria usually associated with an acute MI.
Symptoms of AMI in women may differ from those in men but not markedly. Most important, women more frequently report pain/discomfort in the neck or jaw and back, as well as nausea; they score their pain/discomfort slightly higher than men (Thuresson et al. 2005).
In younger women aged 35–55 presenting with severe but somewhat atypical chest pain precipitated by an acute stressful event, stress cardiomyopathy (Takotsubo cardiomyopathy) should be excluded by an urgent echocardiographic assessment (see Fig. 11-2).
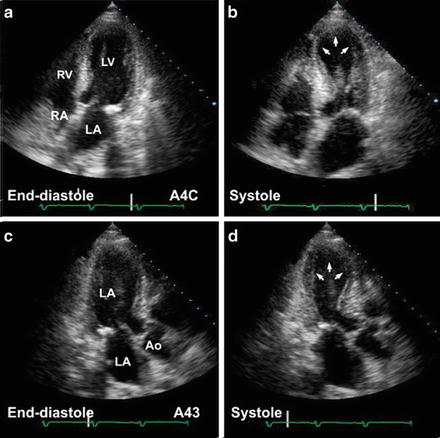
Fig. 11-2.
Transient myocardial stunning: stress-related Takotsubo: transient left apical ballooning. Apical four-chamber views in this 62-year-old female who presented with chest pains show (a, b) akinetic apical segments, markedly hypokinetic mid-ventricular segments, with preserved basal segments—a pattern not consistent with coronary artery anatomy. Work-up for acute myocardial infarct—enzymes, electrocardiogram, and cardiac catheterization—was nondiagnostic. Follow-up echocardiogram 6 weeks later (c, d) showed normal cardiac function (Reproduced with permission from Bulwer BE, Solomon SD (2007) Cardiomyopathies. In: Solomon SD, Bulwer BE, editors. Essential Echocardiography: A Practical Handbook. Totowa, NJ: Humana Press, 2007; p. 187. With kind permission from Springer Science + Business Media.)
Astute observation of the electrocardiographic (ECG) changes of STEMI remains crucial for diagnosis and must be mastered to command rapid commencement of PCI or thrombolytic therapy.
STEMI can be diagnosed within 10–30 min by observation of ST-segment changes in two or three serial ECGs. The ECG is the crucial test needed for diagnosis. Troponins or MRI are second runners because time is of the essence.
Troponin levels are important in defining high-risk NSTEMI, but have no role to play in diagnosis of STEMI prior to PCI done within 90 min of arrival at a PCI facility. It is also of no value in areas where only thrombolytic therapy is available and can be given during ambulance transport or within 20 min of arrival at an emergency room.
Serial changes in highly sensitive troponin I assay and earlier diagnosis of MI may evolve (Keller et al. 2011). But there are many other conditions that can cause troponin elevation: Causes of elevated troponin values in clinical settings other than acute MI (White and Chew 2008) include: tachyarrhythmia, bradyarrhythmia, heart block, severe hypertension, cardiogenic shock, hypotension, heart failure, aortic dissection, hypertrophic cardiomyopathy, rhabdomyolysis with cardiac myocyte necrosis, Takotsubo cardiomyopathy, transplant vasculopathy, myopericarditis, rheumatoid arthritis, systemic vasculitis, post-viral infiltrative diseases of the myocardium, amyloidosis, sarcoidosis, hemochromatosis, scleroderma, atrioventricular ablation, defibrillation, chest wall trauma, cardiac surgery renal failure, transient ischaemic attack, stroke, or subarachnoid hemorrhage, drug toxicity (e.g., adriamycin, 5-fluorouracil, daunorubicin, herceptin, etc.), hypothyroidism pulmonary embolism, severe asthma, pulmonary hypertension, sepsis (including sepsis occurring with shock), critically ill patients, pheochromocytoma, severe burns, kawasaki disease, extreme exertion, and snake venom.
CK-MB still has a role for the diagnosis of NSTEMI where troponin estimation is not available.
The ECG remains unchallenged, therefore, for the diagnosis of STEMI which by definition engages abnormal elevation of the ST segment.
Electrocardiographic Features
Although sophisticated tests have evolved to improve diagnostic accuracy, they are of limited value in the era of aggressive PCI and thrombolysis. Thus, a rapid relevant history and correct interpretation of the ECG repeated within minutes are of paramount importance in the implementation of early PCI, or thrombolytic therapy, either of which will be of greatest benefit if instituted within 2 h of symptom onset.
Inferior MI Diagnosis
Abnormal contour ST-segment elevation of more than 1 mm (0.1 mV) in two or more contiguous inferior leads II, III, and aVF. Figures 11-3 and 11-4 reveal abnormally coved ST-segment elevation in inferior leads with reciprocal depression in leads I and AVL diagnostic of inferior STEMI. Note the reciprocal depression in 1, AVL, V1–V3
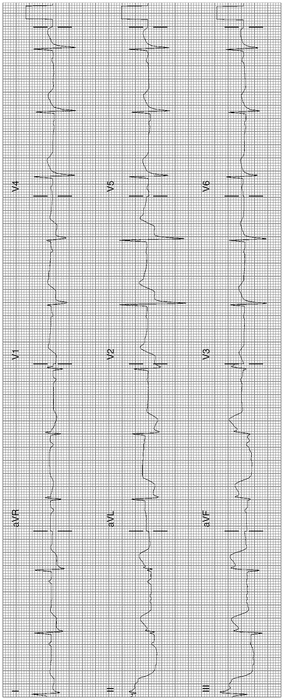
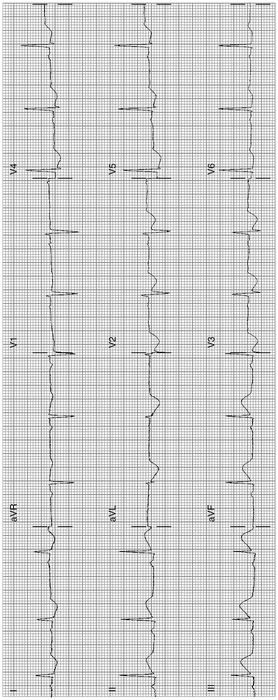

Fig. 11-3.
Acute inferior MI (STEMI); abnormally shaped high ST-segment elevation in inferior leads. Note the reciprocal depression in leads I, aVL, V1, V2. Reciprocal ST-segment depression strengthens the diagnosis of acute MI (Reproduced with permission from Khan M Gabriel, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer; 2011. With kind permission from Springer.)

Fig. 11-4.
ST elevation is less than that shown in Fig. 11.3, but the ST contour is frankly abnormal in inferior leads; note in both Figs. 4 and 5 the ST elevation in lead III is greater than that seen in lead 2, a typical feature of inferior MI. ST-segment reciprocal depression in 1, aVL, V2 V3 (Reproduced with permission from Khan M Gabriel, Encyclopedia of Heart Diseases, 2nd edn. New York: Springer; 2011. With kind permission from Springer.)
Anterior MI Diagnosis
Greater than 1 mm (0.1 mV) abnormal contour ST-segment elevation in two or more contiguous precordial leads. But leads V2 and V3 often show some ST elevation in normal men and the following adjustment is advisable: V2 and V3 should be >2 mm in men; >1.5 mm in women to be considered significant; note ST elevation should be preferably in three contiguous precordial leads: thus, elevation in V1–V3; or V2–V4. This caution may avoid administration of thrombolytic agents or coronary angiograms to individuals without MI.
Figure 11-5a shows anteroseptal MI; Fig. 11-5b shows marked ST elevation V2–V5, lead I aVL, and Q-waves, diagnostic of acute anterior MI.
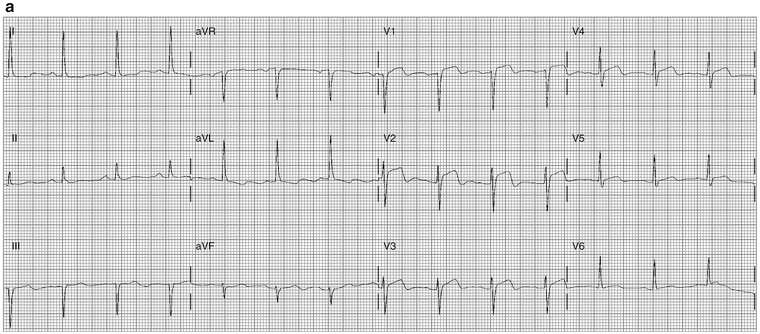
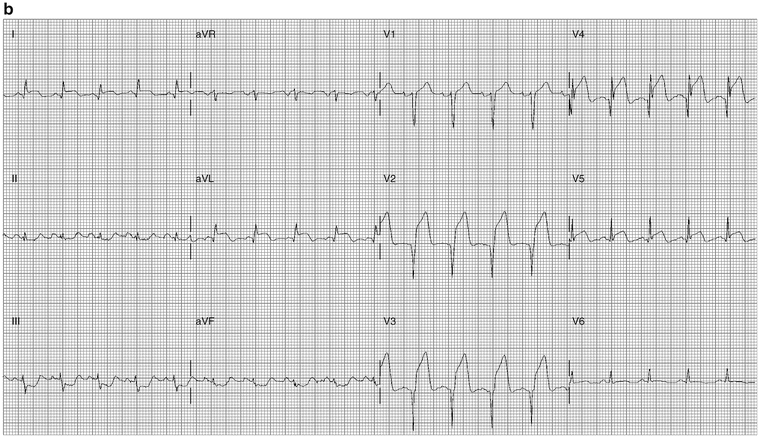


Fig. 11-5.
(a) Abnormal coving, typical features of anterior myocardial injury, STEMI. (b) ECG from a 39-year-old male with ST-segment elevation V2–V5: acute anterior MI (Reproduced with permission from Khan M Gabriel, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer; 2011. With kind permission from Springer.)
Where symptoms are not typical, the response to nitroglycerin must be ascertained. Also, minimal ST-segment elevation in black patients, other ethnic groups, and athletes must be reassessed to exclude the occasional normal variant.
ECG Mimics of Acute Myocardial Infarction
Normal Variants: see Figs. 11-6 and 11-7 which show so-called early repolarization pattern
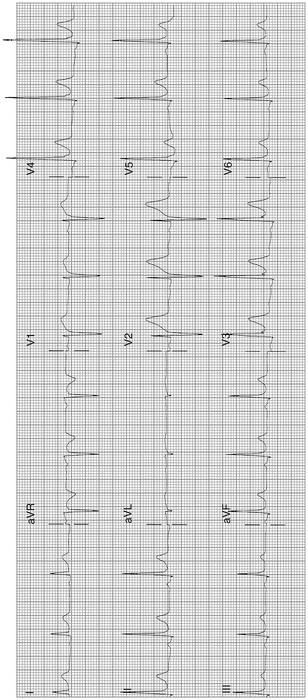
Fig. 11-6.
ECG from a 25-year-old male: ST elevation 1.5 mm in leads II, III, and aVF; also, elevation 2 mm in V2–V4, but normal curve, and fishhook in V3: typical normal variant (Reproduced with permission from Khan M Gabriel, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer; 2011. With kind permission from Springer.)
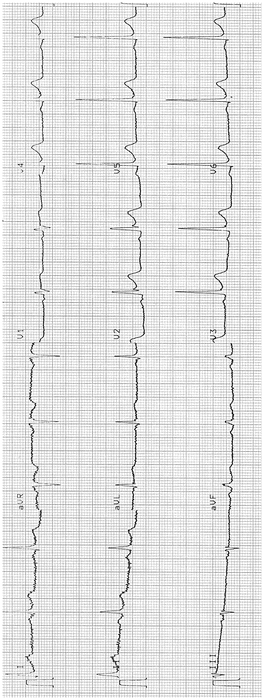
Fig. 11-7.
ECG from a 45-year-old male: ST elevation 2 mm in V2, V3; fishhook in V2 (Reproduced with permission from Khan M Gabriel, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer; 2011. With kind permission from Springer.)
Acute Pericarditis; see Fig. 11-8
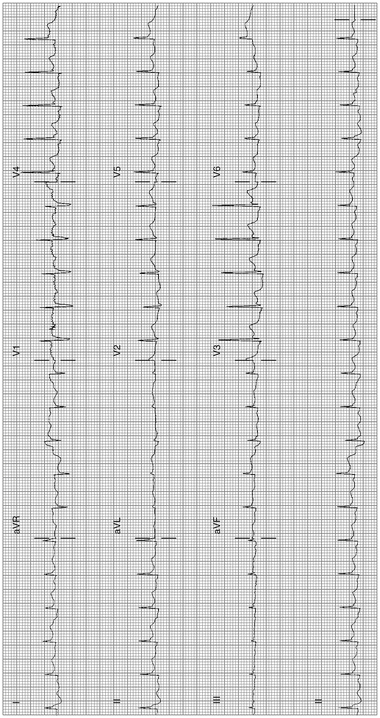
Fig. 11-8.
Acute pericarditis: sinus tachycardia 126/min; typical features: widespread ST-elevation leads I, II, III, aVF, V3–V6. Note aVR shows ST-segment depression and PR-segment elevation. In addition, the J-point level almost equals the height of the T-wave in V6 (Reproduced with permission from Khan M Gabriel, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer; 2011. With kind permission from Springer.)
MI in recent past, age indeterminate; see Fig. 11-9
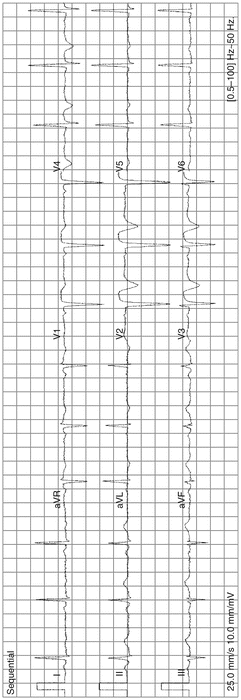
Fig. 11-9.
QS in V1 V2; abnormal ST elevation V1–V3, with T inversion: anteroseptal MI age indeterminate; comparison with old ECG and clinical correlation needed; note absence of reciprocal depression as not acute MI (Reproduced with permission from Khan M Gabriel, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer; 2011. With kind permission from Springer.)
Left bundle branch block: see Fig. 11-10. The V leads commonly show small r-waves in V1, V2, or QS complexes; poor R-wave progression with ST elevation V 1–V 3 that can be misinterpreted as an anteroseptal infarct if the interpreter fails to note the QRS duration greater than 0.12 s. LBBB caused by acute MI is a diagnostic problem
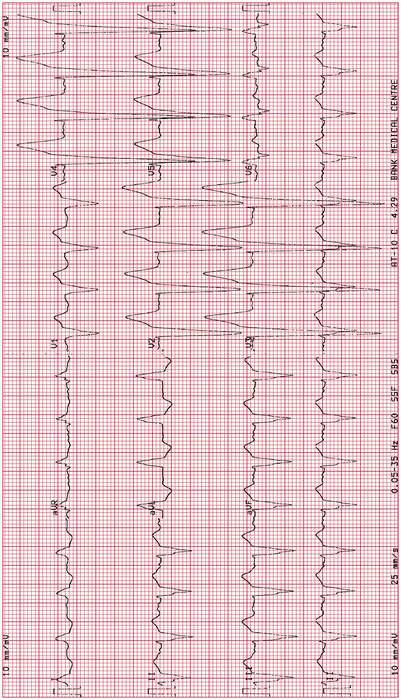
Fig. 11-10.
Poor R-wave progression with ST elevation V2, V3, suggestive of anteroseptal infarction; other features of left bundle branch block (Reproduced with permission from Khan M Gabriel, Rapid ECG Interpretation, 3rd edition. New York: Humana Press/Springer; 2008, p. 347. With kind permission from Springer.)
Stress cardiomyopathy (Takotsubo cardiomyopathy, Fig. 11-2)
Brugada syndrome; see Fig. 11-11
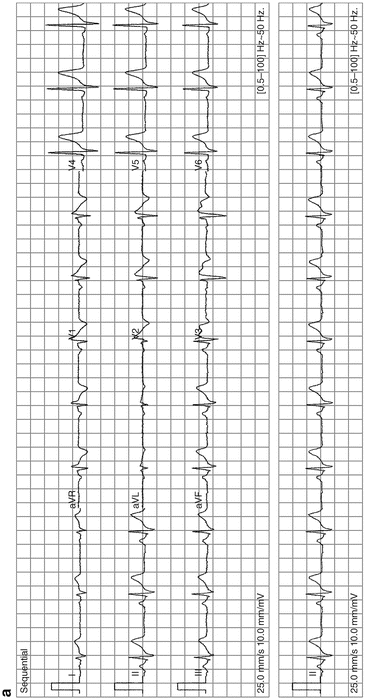
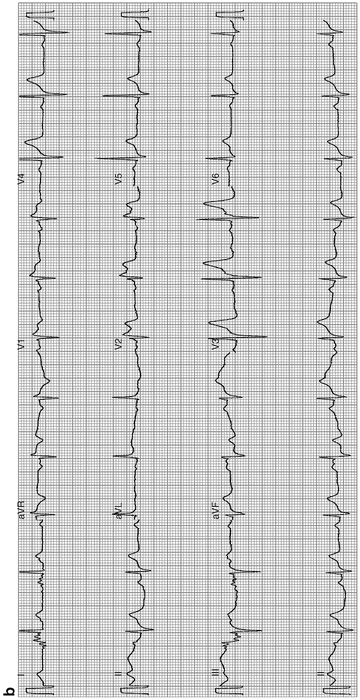
Fig. 11-11.
(a) Typical features of Brugada syndrome. Atypical, incomplete right bundle-branch block (RBBB) with a curious (odd shape) ST-segment elevation/deformity in V1–V3, described as the coved (in V1, V2) and saddle-back patterns (V3). Note there is no widened S-wave in lead 1 V5 or in V6, as seen in true incomplete or complete RBBB. Thus, if you recognize an atypical RBBB think of Brugada syndrome and reassess for the characteristic features of this rare but important diagnosis. ECG from a 40-year-old man with episodes of syncope/collapse. No recurrence of syncope over Fig. 11-11. (Continued) 3 years following ICD. (b) Brugada syndrome: ST elevation V1–V3: rSR1 in V1–V2 suggests incomplete RBBB, but there is no slurred or widened S-wave (the S-wave is not of prolonged duration) in lead I, V5, or V6 to indicate true RBBB or IRBBB. This should alert the interpreter to assess for atypical RBBB, a feature of Brugada syndrome. Scrutiny of the ST segment in V1 and V2 reveals a coved and saddle-back deformity, characteristic features of the syndrome (Reproduced with permission from Khan M Gabriel, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer; 2011. With kind permission from Springer.)
Other causes of ST-segment elevation include:
Prinzmetal’s (Variant) Angina
This disorder is caused by coronary artery spasm. In this uncommon condition, transient ST elevation occurs during pain and resolves with relief of pain or with the administration of nitroglycerin.
Hyperkalemia can cause ST elevation and, rarely, transient Q-waves.
Hypothermia with rectal temperatures below 93 °F (34 °C) may cause distortion of the earliest stage of repolarization; the ST segment becomes elevated in a curious “hitched up” pattern
Primary or secondary tumors may cause ST elevation and Q-waves
Acute myocarditis may present with ST elevation with or without Q-waves (Myocarditis)
Chagasic myocarditis can cause ST and Q-wave changes (Chagas Disease)
Subarachnoid hemorrhage or intracranial hemorrhage may cause ST-segment shifts or alteration of the QT
Therapy
Aspirin: 240–324 mg (3–4 tabs, 75–81 mg) chewed and swallowed at onset of pain caused by a probable heart attack. UK: Aspirin 300 mg (chewed or dispersed in water) is recommended
Prophylaxis: 75–81 mg (non enteric coated) daily after a meal for post-MI, ischemic heart disease, or for others with atherosclerotic disease. See Chaps. 10 and 19 for aspirin pseudoresistance and reason for using soft/chewable aspirin
Clopidogrel 600 mg loading dose; note: morphine decreases effectiveness (Hobl et al. 2014)
Beta-blockers: metoprolol; if LV dysfunction advise carvedilol, bisoprolol, or metoprolol succinate sustained release). Avoid atenolol (see Chaps. 1 and 2)
PCI, which if readily available, is the first choice for reperfusion
Statins at high dose; rosuvastatin 20–40 mg or atorvastatin 60–80 mg daily to maintain LDL-C levels <1.8 (70 mg/dL mmol/L)
Nitrates should not be used by patients who have received a phosphodiesterase inhibitor for erectile dysfunction within the last 24 h (48 h for tadalafil)
Acute MI is usually caused by occlusion of a coronary artery by thrombosis (DeWood et al. 1980) overlying a fissured or ruptured atheromatous plaque. The contents of a ruptured plaque are highly thrombogenic, and exposed collagen provokes platelet aggregation. Thus, the efficacy of aspirin should not be underestimated. Aspirin administered at the onset of symptoms markedly improves survival. In the Second International Study of Infarct Survival (ISIS-2 1988), aspirin caused a 32 % reduction in the 35-days vascular mortality rate. Thus, all patients with known coronary heart disease must be strongly advised to chew and swallow this life-saving agent if chest discomfort exceeds 10 min or if chest pain is not relieved by nitroglycerin (glyceryl trinitrate).
Patients and the public must be informed that the use of 160–325 mg chewable tablets (not enteric coated) taken at the onset of a heart attack can cause a 20 % decrease in the incidence of heart attack or death. Aspirin should be taken once the decision has been made to proceed to the nearest emergency room. Individuals over age 40 (the MI age) should be warned that chewable aspirin acts rapidly and prevents fatal and nonfatal MI, but that nitroglycerin does neither. Nitroglycerin is effective mainly for coronary artery spasm, which is indeed a rare cause of acute MI
In the United Kingdom advice for aspirin is as follows: chewed or dispersed in water at a dose of 150–300 mg. If aspirin is given before arrival at hospital, a note saying that it has been given should be sent with the patient
Patients may be motivated to use this strategy if they are informed that aspirin is more important than the use of nitroglycerin because nitroglycerin may relieve pain of mild angina but does not prevent a heart attack or save lives. If chewable aspirin has not been used, then the dose should be given immediately on arrival at an emergency room
Aspirin does not block catecholamine-induced platelet aggregation and does not decrease the incidence of sudden cardiac death or the occurrence of early-morning AMI. Beta-blocking drugs have good effects here. The combination of aspirin and beta-blockers is life-saving and has proved to be effective.
Pain Relief
Pain relief must be achieved immediately and completely. Pain precipitates and aggravates autonomic disturbances, which may cause arrhythmias, hypotension, or hypertension, thus increasing the size of infarction.
1.
Morphine Dosage: Initial dose of 4–8 mg IV at a rate of 1 mg/min repeated if necessary at a dose of 2–4 mg at intervals of 5–15 min until pain is relieved. The dose is reduced or morphine is discontinued if toxicity is observed, that is, depression of respiration, hypotension, or severe vomiting. The drug allays anxiety, relieves pain, causes venodilation, and therefore reduces preload. In addition, the drug has a favorable effect on VF threshold.
Caution: Morphine has been shown to decrease clopidogrel absorption, decrease concentrations of clopidogrel active metabolite, and diminish its salutary effects. This can cause treatment failure in susceptible patients (Hobl et al. 2014). The drug is avoided or is used under close supervision if severe respiratory insufficiency is present. Severe vomiting and occasionally aspiration may increase cardiac work. Bradycardia is occasionally made worse, so care is required in patients with inferior MI in whom intense vagotonia already exists. Respiratory depression can be treated with the narcotic antagonist naloxone (Narcan) at a dose of 0.4–0.8 mg every 10–15 min as necessary to a maximum of 1.2 mg. Nausea and vomiting can be suppressed by IV metoclopramide 5–10 mg. The antiemetic should be given 5–15 min before the second injection of morphine.
The dose of the antiemetic is titrated to avoid sinus tachycardia.
Diamorphine: In the UK the pain and anxiety of AMI are managed with slow IV injection of diamorphine: 2–5 mg (over 1–2 mg/min), followed by a further 2.5–5 mg if necessary every 4 h. Reduce dose by half for elderly or frail patients.
The drug appears to have a more euphoriant effect than morphine and is preferred physicians in the United Kingdom. An antiemetic such as metoclopramide (if LV function is not compromised) or cyclizine by intravenous injection.
2.
Beta-blockers must be given a more important place in the management of chest pain resulting from MI. Beta-blockers can be considered important second-line agents for the control of ischemic pain. This is of particular importance in patients with anterior infarction accompanied by sinus tachycardia and systolic blood pressure (BP) >110 mmHg. Dramatic pain relief and reduction of ST-segment elevation can be obtained by the administration of a beta-blocking agent. The requirement for opiates is thus reduced. In some patients, pain has been documented to be relieved by the administration of beta-blockers without concomitant use of opiates. Pain may be relieved even in the absence of sympathetic overactivity. Beta-blocking agents. Administer a beta-blocker as soon as possible if it is not contraindicated. Beta-blockers (metoprolol, bisoprolol, carvedilol) given within the first 3 h of onset of chest pain improve survival provided they are used orally in patients with stable BP (systolic >105 mmHg) and there is no evidence of heart failure (HF).
Caution is needed because beta-blockers may increase cardiac mortality when used indiscriminately in patients in whom the drugs are contraindicated, particularly IV use in patients who are hemodynamically unstable, with impending cardiogenic shock, pulmonary edema, or acute MI with HF as was done in COMMIT/Second Chinese Cardiac Study (CCS–2; 2005).
In patients with anterior Killip class less than or equal to 11 ST elevation MI undergoing PCI, early IV metoprolol before reperfusion resulted in higher long-tem left ventricular ejection fraction. This administration reduced the incidence of severe left ventricular dysfunction and implantable cardioverter defibrillator indications and fewer admissions for heart failure (Pizarro et al. 2014). Carvedilol proved beneficial in the Carvedilol Postinfarct Survival Controlled Evaluation (CAPRICORN) study (2001) in patients with ejection fraction (EF) <40 %.
Percutaneous Coronary Intervention
Policies are being mustered at all levels to ensure a door-to-balloon time of <90 min. Unfortunately, despite active public education, triage in the United States, and in many countries, remains difficult, particularly because only approx. 50 % of patients with STEMI reach emergency rooms by ambulance.
PCI performed within the first 2 h of onset of symptoms markedly improves survival and morbidity. Although improvement in survival has been shown in clinical trials to occur with thrombolytic therapy given up to 4 h after the onset of symptoms, the gain is greatest within the first 2 h and then falls off dramatically after the fourth hour. For those who cannot reach a coronary intervention within 2 h or facilities are not available, thrombolytic therapy administered at the earliest moment (<2 h) is of the utmost importance. The delay in the emergency room from the arrival of the patient to the administration of thrombolytic therapy varies from 30 to 90 min. The door-to-needle time is stated to vary widely among hospitals. A delay beyond 20 min is inexcusable.
Extensive public education is essential. This is especially important in patients with known CAD. The patient and relatives must be aware of the early symptoms and signs of AMI. The patient may therefore quickly summon transport by a mobile emergency service that should be equipped with semiautomated defibrillators and provide the use of life-saving thrombolytic therapy; in the absence of such facilities, the patient should present without delay to the nearest emergency room for early therapy which should be given within the golden 1 h
Clopidogrel
Antiplatelet therapy should be initiated before diagnostic angiography with clopidogrel (loading dose 600 mg followed by daily maintenance dose 75 mg).
Morphine has been shown to decrease clopidogrel absorption, decrease concentrations of clopidogrel active metabolite, and diminish its salutary effects. This can cause treatment failure in susceptible patients (Hobl et al. 2014).
Studies have suggested that adverse cardiovascular outcomes with the combination of clopidogrel and a proton pump inhibitor (PPI) are explained by the individual PPI. The use of a PPI inhibits CYP450 2C19, including omeprazole, lansoprazole, or rabeprazole. In particular, omeprazole has been reported to significantly decrease the inhibitory effect of clopidogrel on platelet aggregation. A study reported that the PPI pantoprazole was not associated with recurrent MI among patients receiving clopidogrel, possibly due to pantoprazole’s lack of inhibition of CYP450 2C19 (Juurlink 2009)
Prasugrel
The pharmacokinetic and pharmacodynamic advantages of prasugrel over clopidogrel is clinically important when pretreatment is not possible, and fast inhibition of platelet aggregation is desirable for primary PCI for STEMI (Montalescot et al. 2009). “A loading dose of 60 mg prasugrel achieves faster, more consistent, and greater inhibition of ADP-induced platelet aggregation than does 600 mg of clopidogrel, with a significant effect seen 30 min after administration of prasugrel when no effect is detectable with clopidogrel” (Wiviott et al. 2007; Wallentin et al. 2008).
TRITON-TIMI 38 (2009), a double-blind, randomized controlled trial, compared prasugrel with clopidogrel in patients undergoing PCI for STEMI (Montalescot et al. 2009). See Chaps. 19 and 24.
Prasugrel must not be administered to patients with a history of TIA or stroke. Patients <60 kg have an increased exposure to the active metabolite and an increased risk of bleeding on a 10-mg once-daily dose. It is advisable to lower the maintenance dose to 5 mg in patients who weigh <60 kg, although the effectiveness and safety of the 5-mg dose have not been studied prospectively (Wright et al. 2011).
In the UK: Indications in combination with aspirin for the prevention of atherothrombotic events in patients with ACS undergoing PCI (with aspirin—initially 60 mg as a single dose and then body weight over 60 kg, 10 mg once daily, or body weight under 60 kg or elderly, 5 mg once daily).
Ticagrelor (BRILINTA; Brilique in UK)
Ticagrelor is an oral non-thienopyridine cyclo-pentyl triazolo-pyrimidine ADP-receptor (P2Y12) antagonist, which like prasugrel is more potent and rapid acting than clopidogrel.
Cannon and colleagues for the Platelet Inhibition and Patient Outcomes (PLATO) investigators compared ticagrelor with clopidogrel. Subjects were 13,408 (72 %) of 18,624 patients hospitalized for STEMI and NSTEMI. At 1 year, the primary composite end point occurred in fewer patients in the 6,732 ticagrelor-treated group than in the 6,676 clopidogrel group: p = 0.0025. Ticagrelor compared with clopidogrel significantly reduced all-cause mortality at 12 months in all patients (4.5 % vs. 5.9 %, hazard ratio 0.78, p < 0.001).
Unlike clopidogrel and prasugrel, which bind irreversibly to the platelet surface-membrane (P2Y12) receptor, ticagrelor is a reversible P2Y12 receptor blocker, with platelet function returning to normal, 2–3 days after discontinuation (Gurbel et al. 2009) compared with 5–10 days after discontinuation of clopidogrel and prasugrel. Within 30 min, a ticagrelor loading dose of 180 mg results in roughly the same level of inhibition of platelet aggregation as that achieved 8 h after a clopidogrel loading dose of 600 mg (Gurbel et al. 2009).
Ticagrelor, compared with clopidogrel, reduced the composite of cardiovascular death, MI, and stroke in patients with extensive coronary heart disease (14.9 % vs. 17.6 %) similar to its reduction in those without extensive CAD (6.8 % vs. 8.0 %) (Kotsia et al. 2014).
Bivalirudin: Gregg et al. (2011) was a prospective, open-label, randomized trial in patients with acute STEMI, within 12 h after the onset of symptoms, and who were undergoing PCI (Stone et al. 2011).
Results at 3 years: Compared with 1,802 patients allocated to receive heparin plus a GPI, 1,800 patients allocated to bivalirudin monotherapy had lower rates of all-cause mortality (5.9 % vs. 7.7 %, p = 0.03), cardiac mortality (2.9 % vs. 5.1 %, p = 0.001), and reinfarction (6.2 % vs. 8.2 %, p = 0.04). Major bleeding not related to bypass graft surgery was significantly reduced (6.9 % vs. 10.5 %, p = 0.0001) (Stone et al. 2011).
Bivalirudin in ISAR-REACT 4 Trial: Abciximab and Heparin vs. Bivalirudin for Non-ST-Elevation Myocardial Infarction. Bivalirudin was administered to 860 and abciximab to 861 patients. A strategy of early invasive intervention (within 24 h after admission) was the standard of care at all centers for patients presenting with an ACS and elevated biomarker levels. All the patients in the trial were given 325–500 mg of aspirin and 600 mg of clopidogrel before they received a study drug. There were no significant differences in deaths or recurrent MI.
A deferred angioplasty strategy of nonculprit lesions should remain the standard approach in patients with STEMI undergoing primary PCI, as multivessel PCI may be associated with a greater hazard for mortality and stent thrombosis (Kornowski et al. 2006).
Rivaroxaban: In ATLAS ACS 2–TIMI 51 patients received the first dose of rivaroxaban no sooner than 4 h after the final dose of IV heparin, 2 h after the final dose of bivalirudin, and 12 h after the final dose of other intravenous or subcutaneous enoxaparin or fondaparinux. Patients were randomly assigned to twice-daily administration of either 2.5 or 5.0 mg of rivaroxaban or placebo, with a maximum follow-up of 31 months. Rivaroxaban significantly reduced the primary efficacy end point of death from cardiovascular causes, MI, or stroke, as compared with placebo. The 2.5 mg twice daily achieved efficacy without increased life-threatening bleeding (Mega et al. 2012). There was no significant difference in the rates of fatal bleeding associated with rivaroxaban as compared with placebo (0.3 % vs. 0.2 %, P = 0.66). Rivaroxaban significantly increased the rate of TIMI major bleeding that was not related to CABG, as compared with placebo, with rates of 2.1 and 0.6 %, respectively (hazard ratio, 3.96; 95 % CI, 2.46–6.38; P < 0.001), TIMI bleeding requiring medical attention (14.5 % vs. 7.5 %, P = <0.001), and intracranial hemorrhage (0.6 % vs. 0.2 %, P = 0.009).
The Role of Residual Thrombus was emphasized by Applegate (2011). Rivaroxaban should have a role in the prevention of residual thrombus post-PCI or following thrombolytic therapy or fondaparinux administration.
Thrombolytic Therapy
Although improvement in survival has been shown in clinical trials to occur with thrombolytic therapy given up to 6 h after the onset of symptoms, the gain is greatest within the first 2 h and then falls off dramatically after the fourth hour. Thrombolytic therapy administered at the earliest moment (<2 h) is of the utmost importance. Lives saved / 1000 treated: within 1, 3 and 7 hours respectively: 65 %, 27 %, 8 %.
The delay in the emergency room from the arrival of the patient to the administration of thrombolytic therapy varies from 30 to 90 min. The door-to-needle time is stated to vary widely among hospitals. A delay beyond 20 min is inexcusable. Delays may result from duplication of assessments by different teams of clinicians. Thrombolytic therapy should be administered in the emergency room. The emergency room physician and assistants should have the training and authority necessary to administer a thrombolytic agent.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


